Figure 1. Sensitized phenotyping screen.
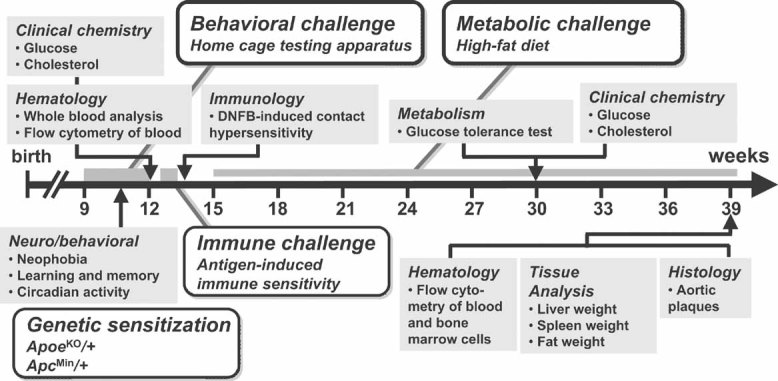
Animals were subjected to a battery of tests designed to reveal effects of gene dosage on physiological parameters (grey boxes) relevant to common human diseases in five therapeutic areas: behaviour, metabolic syndrome, immune dysfunction, atherosclerosis, and cancer. Each therapeutic area was accompanied by a sensitizing environmental, pharmacological or genetic challenge (open boxes). The first cohort of mice were ApoeKO/+ and were subjected to the full phenotyping battery, while a second cohort were ApcMin/+ and were only tested for intestinal neoplasia-induced death. At 9 weeks of age, animals were tested for neophobia, gross activity and spatial learning in a home cage behavioural testing apparatus. At 12 weeks, blood parameters (cell counts and flow cytometry, cholesterol, glucose) were measured and at 14 weeks animals were tested for DNFB-induced CHS. At 15 weeks, animals were placed on a high-fat diet, and after 15 weeks of high-fat diet, blood parameters for clinical chemistry were collected and glucose tolerance measured. After 24 weeks of high fat diet, mice were sacrificed and organs collected. Aortic samples were examined for atherosclerotic plaques and bone marrow cells were extracted and analysed by flow cytometry (all dates are ±2 weeks).
