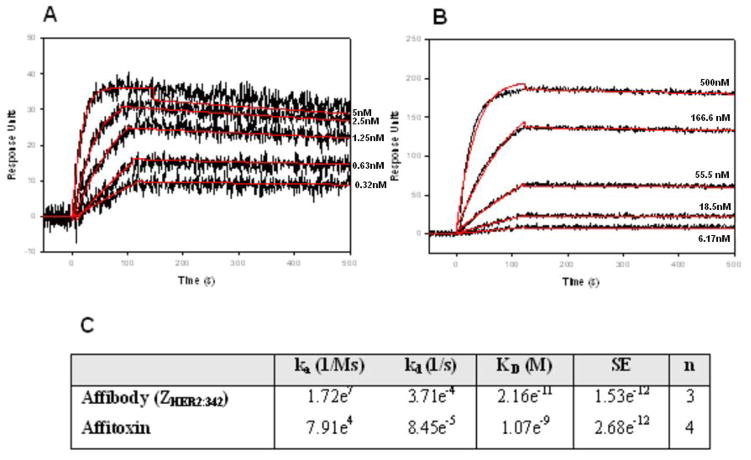
Figure 3. Kinetic analysis of ZHER2:342 Affibody molecule (A) or Affitoxin (B) binding to HER2/Fc-covered surface.
Binding of 0.32, 0.63, 1.25, 2.5, 5 nM wild type ZHER2:342 Affibody molecule (A) and 6.17, 18.5, 55.5, 166.6, 500 nM of Affitoxin (B) to HER2/Fc on the sensor chip was tested using SPR-based binding assay. The red lines represent a global analysis of data using a Langmuir binding model. The Affibody molecule-HER2/Fc complex was allowed to dissociate for 2000 seconds in order to observe a measurable decayKinetic analysis of ZHER2:342 Affibody molecule (A) or Affitoxin (B) binding to HER2/Fc-covered surface. Kinetic data are summarized in C.