Figure 5. Potential role for LSF in mediating signal transduction from APP in the nucleus.
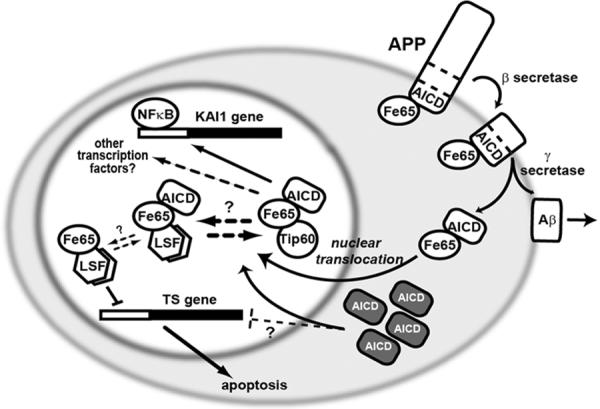
The intracellular domain of the transmembrane protein APP (amyloid precursor protein) interacts with a number of signaling proteins, including Fe65. APP cleavages produce AICD (APP intracellular C-terminal domain) and Aβ (β amyloid) peptide. Aβ is released extracellularly and is responsible for plaque formation in Alzheimer's disease. AICD is released intracellularly, where Fe65 facilitates its translocation into the nucleus. Nuclear Fe65-AICD interacts with Tip60 and LSF, perhaps in competition with each other, as indicated. Fe65 alone inhibits LSF transactivation on the TS promoter, although whether the ternary complex with AICD is also inhibitory is not known. One target of the Fe65-AICD-Tip60 complex is the tetraspanin KAI1 gene, although many other target genes are likely, including through factors other than NF-κB. Overexpression of AICD (dark boxes) increased neuronal apoptosis, presumably in part by increased nuclear translocation and misregulation of target genes, perhaps including TS.
