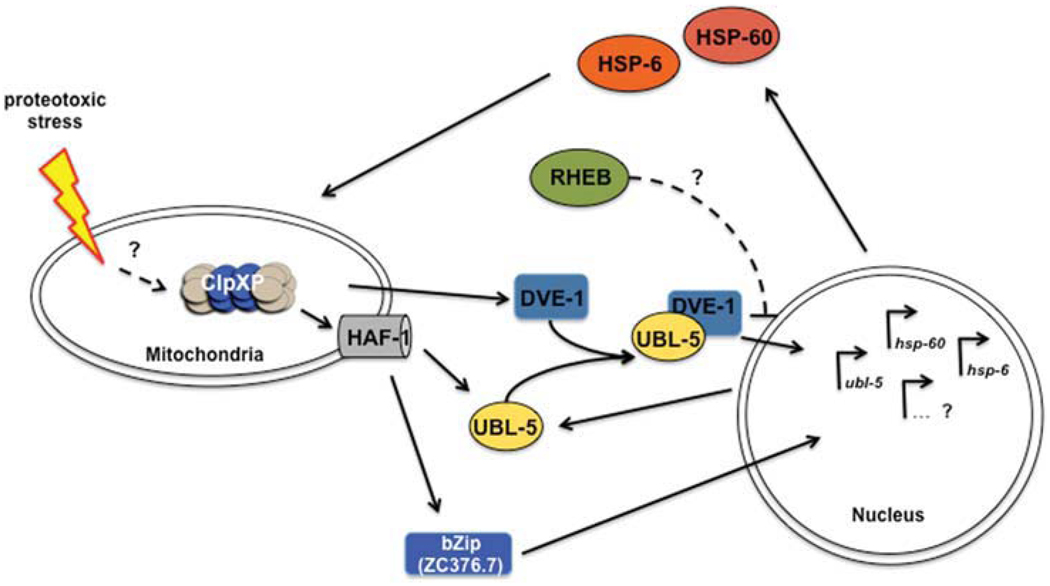
Fig. 3.
Model of the mitochondrial unfolded protein response (UPRmt) in C. elegans. Proteotoxic stress triggers a response of ClpXP, whose proteolytic activity is required to transmit the signal to the cytoplasm presumably via peptide translocation by HAF-1. Here, the transcription factors, UBL-5 and DVE-1, which form a complex, and ZC376.7 redistribute to the nucleus. DVE-1 binds to the promoter region of the mitochondrial chaperone genes, hsp-6 and hsp-60. The expression of ubl-5 is also up-regulated and probably acts as a feed-forward regulation to enhance the signal. This activation of the UPRmt results in an accumulation of HSP-6 (mt Hsp70) and HSP-60 (mt chaperonin) in the mitochondria. RHEB is thought to act as a suppressor of the UPRmt, probably with cessation of the stress conditions. Open questions of the regulation of the UPRmt are indicated with question marks and are further discussed in the text.