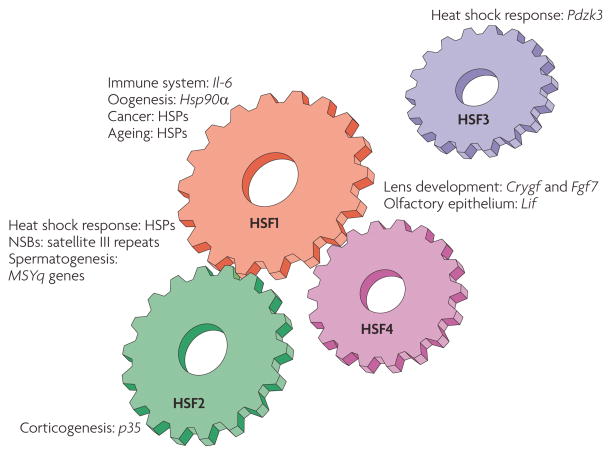
Figure 1. The mammalian HSF machinery.
An overview of the mammalian heat shock factor (HSF) family members and their biological functions. HSFs contribute to multiple normal physiological processes and pathologies through direct regulation of their target genes. The HSF target genes that have been identified in vivo are shown. HSF1 was originally recognized as the principal stress-responsive regulator of the heat shock response, but now HSF2 is known to modulate HSF1-mediated expression of heat shock protein (HSP) genes through heterocomplex formation. On heat shock, HSF1 and HSF2 accumulate into nuclear stress bodies (NSBs), where they bind to satellite III repeats. HSF1 is also a regulator of immune responses and cancer. So far, the regulation of HSP genes in ageing has most intensively been examined in Caenorhabditis elegans. Both HSF1 and HSF2 have been ascribed regulatory functions in several developmental processes, such as oogenesis, spermatogenesis and corticogenesis. HSF4 is involved in the development of different sensory organs in cooperation with HSF1, but has no role in the heat shock response. Murine HSF3 is the most recently identified mammalian HSF, which participates in the heat shock response by binding to the PDZ domain-containing 3 (Pdzk3) promoter10. Currently, HSF3 is not known to crosstalk with any member of the HSF family, and is therefore placed separately from the other HSFs. Crygf, crystallin γF; Fgf7, fibroblast growth factor 7; Il-6, interleukin-6; MSYq, male-specific long arm of the mouse Y chromosome.