Abstract
Background
Since the number of heart failure (HF) patients is still growing and long-term treatment of HF patients is necessary, it is important to initiate effective ways for structural involvement of primary care services in HF management programs. However, evidence on whether and when patients can be referred back to be managed in primary care is lacking.
Aim
To determine whether long-term patient management in primary care, after initial optimisation of pharmacological and non-pharmacological treatment in a specialised HF clinic, is equally effective as long-term management in a specialised HF clinic in terms of guideline adherence and patient compliance.
Method
The study is designed as a randomised, controlled, non-inferiority trial. Two-hundred patients will be randomly assigned to be managed and followed in primary care or in a HFclinic. Patients are eligible to participate if they are (1) clinically stable, (2) optimally up-titrated on medication (according to ESC guidelines) and, (3) have received optimal education and counselling on pre-specified issues regarding HF and its treatment. Furthermore, close cooperation between secondary and primary care in terms of back referral to or consultation of the HF clinic will be provided.The primary outcome will be prescriber adherence and patient compliance with medication after 12 months. Secondary outcomes measures will be readmission rate, mortality, quality of life and patient compliance with other lifestyle changes.
Expected results
The results of the study will add to the understanding of the role of primary care and HF clinics in the long-term follow-up of HF patients.
Keywords: Heart failure, Primary care, Follow-up, Guideline adherence, Patient compliance, Quality of life
Background
Chronic heart failure (HF) represents an emerging epidemic in Western societies [1]. Although treatment of HF has certainly improved in the past decades with the development of multiple medications and devices, mortality and morbidity are still considerable. There is no doubt that adherence to evidence-based drug therapy and lifestyle advice is crucial in optimising prognosis in HF patients [1, 2]. To achieve this, a multidisciplinary approach is advocated including counselling to enhance patient compliance.
Although the COACH study [3] has shown that the optimal model of HF disease management is not known yet, other studies have revealed that multidisciplinary HF disease management programs can be effective in terms of improving patient adherence, decreasing hospital readmission and mortality [4–6] and are now generally accepted as standard care [7–9]. Most of these studies evaluated hospital based (outpatient) disease management. Only a few studies included primary care, and within these studies the intervention was mainly nurse driven. Furthermore, structural involvement of primary care by the general practitioner (GP) is limited in most European countries, with the exception of some of the Western European countries, such as Scotland. In the Netherlands, GPs play a crucial role in 30 % of the HF management programs [8].
With the growing number of HF patients needing treatment and long-term follow-up, it becomes more and more important to look critically at the effective use or different healthcare resources and different models of care. Terminating follow-up does not seem to be a favourable option since studies have shown that after a short intervention or after ending an intervention program the results of the initial optimisation and education will decrease within the next year [10, 11]. The structural involvement of primary care services in HF management programs needs to be initiated moreover, since GPs are able to see patients in their home environment, it may be preferable to incorporate additional follow-up within the primary healthcare system.
Currently, there are no studies assessing whether and when patients can be referred back to the GP to be managed further in primary care. Referral to the GP is more likely to be a viable option in European countries with a strong primary care-based healthcare system with GPs working with high quality primary care guidelines for many chronic diseases [4]. The guideline of the Dutch College of General Practitioners [12] suggests that HF patients can and should be treated and monitored by GPs (in collaboration with primary care nurses) in the primary care setting. On the other hand, treatment and monitoring of HF patients by GPs is described as not optimal [13]. For example, guideline adherence in HF patients primarily treated by their GP was shown to be lower than in those treated by cardiologists [13–17]. These differences can be partly explained by differences in the characteristics of the two patient populations (age, gender and comorbidity), but more importantly, differences may also be attributable to the GPs attitude towards the uptake of treatment. GPs often experience barriers in implementing the prevailing guidelines especially regarding the optimisation of the drug regimen [18, 19]. There are a limited number of studies that have evaluated improvement of treatment skills of general practitioners [20–23]. These studies show that with specific training interventions or with specific specialist recommendations, improvement is possible. Studies that actually compare the long-term treatment and follow-up in the HF clinic with long-term treatment and follow-up in primary care after initial treatment at the HF clinic are not (yet) available. In the NorthStar study [24], Danish researchers test the hypothesis that clinically stable, educated, and medically optimised patients (with NT-proBNP levels < 1000 pg/ml) can be safely managed by the GP.
Within the current study patients will be referred back to the GP in primary care under the following conditions; (1) patients are in a stable condition (no hospital admissions in the previous month, no visits at the emergency unit for decompensation in the previous month, no unplanned medication changes in the previous month), (2) patients are optimally up-titrated on medication according to the current European Guideline on the Diagnosis and Treatment of Chronic Heart Failure [1] and on the Dutch Multidisciplinary Guideline on Chronic Heart Failure [25], (3) patients have received optimal education and counselling on pre-specified issues [26, 27]. Furthermore, close cooperation between secondary and primary care in terms of back referral to or consultation of the HF clinic will be provided as it is an important condition to facilitate optimal follow-up.
The aim of the current study is to determine whether long-term follow-up in primary care, under the above-described conditions, is equally effective as follow-up at a specialised HF clinic in terms of guideline adherence, patient compliance and readmission rates in patients with heart failure.
Methods
Design
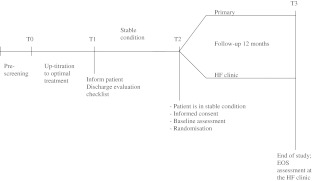
A multicentre, non-inferiority, randomised, controlled trial will be performed. The study complies with the Declaration of Helsinki and is approved by the Central Ethics Committee of the University Medical Hospital Groningen. HF patients visiting the HF clinics of the participating centres will be (pre)screened for their eligibility for the study. Within a period of 3–4 months patients will be up-titrated to optimal medication and educated on HF, its treatment and lifestyle changes. When a stable condition is reached for at least 4 weeks and for a maximum of 2 years (for definition: see below), patients will be randomly allocated to one of two treatment arms: follow-up care by the GP or follow-up care by the specialised HF clinic. Patients will be followed for 12 months (Fig. 1). This trial is listed at www.ntr.nl (NTR1729).
Fig. 1.
Study Design
Study population
Patients are recruited from 4 outpatient HF clinics in the Netherlands: Groningen (UMCG), Ziekenhuis Groep Twente (Almelo and Hengelo), the Deventer Hospital and the Wilhelmina Hospital (Assen).
Inclusion criteria
Patients will be screened and are eligible when they have:
Documented symptoms of HF (either currently or at time of diagnosis);
HF with evidence for structural underlying ventricular dysfunction (left ventricular ejection fraction (LVEF) <45 % at time of diagnosis);
and when they:
Are up-titrated to optimal pharmacological treatment (notably use of adequate dosages of ACE inhibitors/angiotensin receptor blockers(ARBs) and β-blockers);
Have been in a clinically stable condition for at least 1 month and for a maximum of 2 years: no hospital admissions in the previous month, no visits to the emergency unit for decompensation in the previous month, no unplanned medication changes in the previous month;
Are optimally educated and informed on heart failure and the required lifestyle changes following a pre-specified protocol;
Aged above 18 years.
Exclusion criteria
Patients will be excluded from the study when:
Patient management by a cardiologist planned for diagnostics or treatment is needed;
The general practitioner has substantial arguments against patient participation in the study;
The patient has restrictions that render him/her unable to fill in data collection material (inadequate mastering of the Dutch language);
The patient has a life expectancy shorter than 6 months;
The patient is living in a nursing home;
The patient has a current psychiatric disorder as documented in the medical record.
Sample size calculation
The study is designed as a non-inferiority trial. Non-inferiority for guideline adherence will be declared if the lower limit of the one-sided 95 % CI of the difference does not exceed a delta of 20 % from the guideline adherence rate in standard care. Seventy-five patients randomised to receive standard care and 75 patients to receive primary care are needed to demonstrate non-inferiority for guideline adherence assuming a standard care guideline adherence rate of 60 % and a power of 80 %. From earlier research it is known that guideline adherence in primary care is substantially lower (20 % conform the IMPROVEMENT study [13] and Rutten’s study [15] compared with treatment by a cardiologist (60 % conform the MAHLER study [2]. The lower acceptable margin of 40 % has been chosen to provide assurance that the standard care arm of this study has a clinically relevant superiority over historical data. In our point of view this rather wide non-inferiority margin could be justified because the primary care arm has subjective advantages in a number of other aspects when compared with standard care. To ensure the appropriate patient number at the end of the study 2 × 100 patients will be included.
Primary outcome
The primary outcome of the study is guideline adherence defined as the prescription of guideline recommended HF medication (β-blocker and ACE inhibitor/ARB and spironolactone). The global Guideline Adherence Indicator (GAI-3), from the MAHLER study [2], will be used to assess overall guideline adherence. This is a score addressing the relevant groups of medication for heart failure correcting for New York Heart Association (NYHA) class and is quantified for each patient as the proportion of evidence-based recommendations followed by the HF clinic or GP out of the total number of recommendations that applied for that particular patient (Table 1).
Table 1.
Guideline adherence indicator-3
| ACE-I/ARB | Beta blocker | Aldosterone antagonist | GAI-3 (%) | |
|---|---|---|---|---|
| NYHA II | Yes | Yes | - | 50 + 50 |
| NYHA III/IV | Yes | Yes | Yes | 33.3 + 33.3 + 33.3 |
ACE-I ACE inhibitors, ARB angiotensin receptor blockers, GAI Guideline Adherence Indicator, NYHA New York Heart Association
The secondary primary outcome is patient compliance with medication: patient compliance with medication is calculated from digital pharmacy records in terms of the medication possession ratio, e.g. the number of days for which the prescribed medication was available between the last refill in the observation year and the last refill in the foregoing year divided by the number of days between these refills, expressed in a percentage [28].
Secondary endpoints
Secondary endpoints of the study will be guideline adherence regarding medication (optimal dose and adjusted for comorbidity), readmission rate, mortality, (N-terminal) pro-brain natriuretic peptide ((NT-pro)BNP), patient compliance with lifestyle changes and quality of life (Table 2).
Table 2.
Variables and measurements
| Variable | Data collection method |
|---|---|
| Prescribed medication | Chart review/Pharmacy records |
| Patient compliance | Pharmacy records |
| Readmission rate | Chart review |
| Mortality | Chart review |
| (NT-pro)BNP | Blood sample |
| Patient compliance with lifestyle changes | Questionnaires; |
| European Self-Care Behaviour Scale [29] | |
| Revised Heart Failure Compliance Questionnaire [30] | |
| Medication Adherence Report Scale [31] | |
| Weight diary | |
| Heart failure knowledge | Dutch Heart Failure Knowledge Questionnaire [32] |
| Quality of life (QoL) | Questionnaires: |
| SF 36 [33] | |
| Kansas City Cardiomyopathy Questionnaire [34] | |
| EuroQol5D [23, 35, 36] | |
| Demographics | Chart review |
| Medical history | Chart review |
| Comorbid diseases | Chart review |
| NYHA | Chart review |
| LVEF | Chart review |
| Laboratory | Chart review |
| Patient/partner depression | Questionnaires (CES-D [37]) |
| Patient/partner perceived control | Questionnaires (CAS-4 [38]) |
| Patient and partner/family satisfaction | Questionnaires (SF 36 [33], EuroQol5D [23, 36] |
| Caregiver QoL | Questionnaires (Caregiver Reaction Assessment [39] |
| Caregiver tasks and burden | Dutch Objective Burden Inventory [40] |
LVEF left ventricular ejection fraction, NYHA New York Heart Association
During the study, data will be collected on demographics, clinical variables (medical history, time since HF diagnosis, previous admissions, comorbidity, heart rate, ECG, LVEF, NYHA class, RR, laboratory findings) and patient and partner satisfaction with care.
Assessment, randomisation and intervention protocol
Randomisation and assessments
Following confirmation of the patient’s eligibility and after informed consent has been obtained, baseline characteristics of the patient will be assessed from the medical chart and patient questionnaires. After baseline assessment, patients will be randomly allocated in each participating centre to either long-term follow-up in primary care (study group 1) or at the HF clinic (study group 2). Follow-up assessment will be done at the end of study after 12 months. Data will be collected through patient questionnaires and medical charts at the HF clinic or at the GP’s office.
‘Intervention’ protocol
Patients in study group 1 will be followed in primary care. Contacts and visits will take place according to the European Guideline [1] and the recently published Dutch Multidisciplinary Guideline on Chronic Heart Failure [25]. Routine visits to the cardiologist or HF nurse are not scheduled; however, referral back to or consultation of the HF clinic is possible. Patients randomised into study group 2 will be followed at the hospital-based heart failure clinic (cardiologist and HF nurse). Contacts and visits will take place according to the Dutch Multidisciplinary Guideline on Chronic Heart Failure [25]. Contact with the GP will be following the care as usual principal.
Data analysis
The primary analysis will compare differences in guideline adherence at 1 year between the two study groups (relative risk and risk difference with 95 % confidence intervals). For continuous secondary endpoints, comparisons between the two study groups will be made with ANCOVA, adjusted for differences in baseline values, when appropriate. For categorical variables, adequate statistical techniques will be used, with adjustment for baseline values, when appropriate.
Study organisation
Study centres
In order to include 200 patients, 4 hospitals in the Netherlands will participate in the study.
The Steering Committee consists of: M.L. Luttik, RN, PhD, chair and project leader; Prof. T. Jaarsma, RN, PhD; Prof. H.L. Hillege, MD, PhD; Prof. A.W. Hoes MD, PhD; Prof K. van der Meer, MD, PhD; Prof. A.A Voors, MD, PhD; Prof D.J. van Veldhuisen, MD, PhD (principal investigator); G. Linssen, MD; D. Lok, MD; and R.M. de Jong, MD, PhD.
Support and monitoring
The study will be supported by the Trial Coordination Centre (University Medical Center, Groningen, the Netherlands), a contract research organisation for clinical trials. Both the quality of the data and of the intervention will be monitored closely.
Conclusion
The results of the COACH-2 study will add to the understanding of the role of primary care in the long-term follow-up of HF patients. This study is the first to provide data on the effectiveness of long-term treatment of clinically stable HF patients who are on optimal treatment by the GP in the primary care setting. This insight is needed in order to create and assure optimal long-term care for HF patients. Accordingly, this strategy may imply an increased participation of primary care in evidence-base HF management.
Acknowledgments
Funding
The Netherlands Heart Foundation (NHF) financially supports the study as one of their research programs (2008B083).
References
- 1.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Komajda M, Lapuerta P, Hermans N, et al. C. Adherence to guidelines is a predictor of outcome in chronic heart failure: the MAHLER survey. Eur Heart J. 2005;26:1653–1659. doi: 10.1093/eurheartj/ehi251. [DOI] [PubMed] [Google Scholar]
- 3.Jaarsma T, Wal MH, Lesman-Leegte I, et al. Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating StudyEvaluating Outcomes of Advising and Counseling in Heart Failure (COACH) Arch Intern Med. 2008;168:316–324. doi: 10.1001/archinternmed.2007.83. [DOI] [PubMed] [Google Scholar]
- 4.Porte PW, Lok DJ, Veldhuisen DJ, et al. Added value of a physician-and-nurse-directed heart failure clinic: results from the Deventer-Alkmaar heart failure study. Heart. 2007;93:819–825. doi: 10.1136/hrt.2006.095810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gohler A, Januzzi JL, Worrell SS, et al. A systematic meta-analysis of the efficacy and heterogeneity of disease management programs in congestive heart failure. J Card Fail. 2006;12:554–567. doi: 10.1016/j.cardfail.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 6.McAlister FA, Stewart S, Ferrua S, et al. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44:810–819. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 7.Jaarsma T, Stromberg A. Heart failure clinics in Europe. Prog Cardiovasc Nurs. 2000;15:67–68. doi: 10.1111/j.0889-7204.2000.080395.x. [DOI] [PubMed] [Google Scholar]
- 8.Jaarsma T, Tan B, Bos RJ, et al. Heart failure clinics in the Netherlands in 2003. Eur J Cardiovasc Nurs. 2004;3:271–274. doi: 10.1016/j.ejcnurse.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 9.McDonagh TA, Blue L, Clark AL, et al. ESC Heart Failure Association Standards for Delivering Heart Failure Care. Eur J Heart Fail 2010;Dec 15. [Epub ahead of print] [DOI] [PubMed]
- 10.Jaarsma T, Halfens R, Huijer Abu-Saad H, et al. Effects of education and support on self-care and resource utilization in patients with heart failure. Eur Heart J. 1999;20:673–682. doi: 10.1053/euhj.1998.1341. [DOI] [PubMed] [Google Scholar]
- 11.Ojeda S, Anguita M, Delgado M, et al. Short- and long-term results of a programme for the prevention of readmissions and mortality in patients with heart failure: are effects maintained after stopping the programme? Eur J Heart Fail. 2005;7:921–926. doi: 10.1016/j.ejheart.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 12.NHG standaard Hartfalen; eerste herziening. Huisarts en Wetenschap 2009;48:64–76.
- 13.Cleland JG, Cohen-Solal A, Aguilar JC, et al. Management of heart failure in primary care (the IMPROVEMENT of Heart Failure Programme): an international survey. Lancet. 2002;360:1631–1639. doi: 10.1016/S0140-6736(02)11601-1. [DOI] [PubMed] [Google Scholar]
- 14.Bongers FJ, Schellevis FG, Bakx C, et al. Treatment of heart failure in Dutch general practice. BMC Fam Pract. 2006;7:40. doi: 10.1186/1471-2296-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutten FH, Grobbee DE, Hoes AW. Differences between general practitioners and cardiologists in diagnosis and management of heart failure: a survey in every-day practice. Eur J Heart Fail. 2003;5:337–344. doi: 10.1016/S1388-9842(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 16.Bosch M, Wensing M, Bakx JC, et al. Current treatment of chronic heart failure in primary care; still room for improvement. J Eval Clin Pract. 2010;16:644–650. doi: 10.1111/j.1365-2753.2010.01455.x. [DOI] [PubMed] [Google Scholar]
- 17.Dahlstrom U, Hakansson J, Swedberg K, et al. Adequacy of diagnosis and treatment of chronic heart failure in primary health care in Sweden. Eur J Heart Fail. 2009;11:92–98. doi: 10.1093/eurjhf/hfn006. [DOI] [PubMed] [Google Scholar]
- 18.Kasje WN, Denig P, Graeff PA, et al. Perceived barriers for treatment of chronic heart failure in general practice; are they affecting performance? BMC Fam Pract. 2005;6:19. doi: 10.1186/1471-2296-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuat A, Hungin AP, Murhpy JJ. Barriers to accurate diagnosis and effective management of heart failure in primary care: qualitative study. BMJ 2003;326. [DOI] [PMC free article] [PubMed]
- 20.Peters-Klimm F, Muller-Tasch, Remppis A, et al. Improved guideline adherence to pharmacotherapy of chronic systolic heart failure in general practice; results from a cluster randomized controlled trial of implementation of a clinical practice guideline. J Eval ClinPract. 2008;14:823–829. doi: 10.1111/j.1365-2753.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 21.Verdu Rotellar JM, Barroso A, Bernaldez MJ, et al. Beta-blocker treatment of stable heart failure in primary care. Rev Esp Cardiol. 2009;62:1141–1148. doi: 10.1016/S0300-8932(09)72383-8. [DOI] [PubMed] [Google Scholar]
- 22.Schuchtert A, BISEX Investigators Effect of bisoprolol treatment for chronic heart failure initiated and followed up by primary care physicians. Eur J Heart Fail. 2005;7:604–611. doi: 10.1016/j.ejheart.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Guadagnoli E, Normand SL, DiSalvo TG, et al. Effects of treatment recommendations andspecialist intervention on care provided by primary care physicians to patients with myocardial infarction or heart failure. Am J Med. 2004;117:433–435. doi: 10.1016/j.amjmed.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Schou M, Gustafsson F, Videbaek L, et al. Design and methodology of the NorthStar study: NT-proBNP stratified follow-up in outpatient heart failure clinics. A randomized Danish multicenter study. Am Heart J. 2008;156:649–655. doi: 10.1016/j.ahj.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Hoes AW, Voors AA, Rutten FH, et al. NHG standaard Hartfalen. Huisarts & Wetenschap. 2010;7:368–389. [Google Scholar]
- 26.Wal MH, Jaarsma T, Veldhuisen DJ. Non-compliance in patients with heart failure; how can we manage it? Eur J Heart Fail. 2005;7:5–17. doi: 10.1016/j.ejheart.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Wal MH, Jaarsma T, Moser DK, et al. Unraveling the mechanisms for heart failure patients’ beliefs about compliance. Eur Heart J. 2007;36:253–261. doi: 10.1016/j.hrtlng.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Vink NM, Klungel OH, Stolk RP, et al. Comparison of various measures for assessing medication refill adherence using prescription data. Pharmacoepidemiol Drug Saf. 2009;18:159–165. doi: 10.1002/pds.1698. [DOI] [PubMed] [Google Scholar]
- 29.Jaarsma T, Stromberg A, Martensson J, et al. Development and testing of the European Heart Failure Self-Care Behaviour Scale. Eur J Heart Fail. 2003;5:363–370. doi: 10.1016/S1388-9842(02)00253-2. [DOI] [PubMed] [Google Scholar]
- 30.Evangelista LS, Berg J, Dracup K. Relationship between psychosocial variables and compliance in patients with heart failure. Heart Lung. 2001;30:294–301. doi: 10.1067/mhl.2001.116011. [DOI] [PubMed] [Google Scholar]
- 31.Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555–567. doi: 10.1016/S0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 32.Wal MH, Jaarsma T, Moser DK, et al. Development and testing of the Dutch Heart Failure Knowledge Scale. Eur J Cardiovasc Nurs. 2005;4:273–277. doi: 10.1016/j.ejcnurse.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Ware JE, Kosinski M, Keller SD. SF-36 physical and mental health. Health Survey, Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Centre; 1994. [Google Scholar]
- 34.Green CP, Porter CB, Bresnahan DR, et al. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/S0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 35.EuroQol--a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy 1990;16:199–208. [DOI] [PubMed]
- 36.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 37.Radloff L. A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 38.Moser DK, Dracup K. Psychosocial recovery from a cardiac event: The influence of perceived control. Heart & Lung. 1995;4:273–280. doi: 10.1016/s0147-9563(05)80070-6. [DOI] [PubMed] [Google Scholar]
- 39.Given CW, Given B, Stommel M, et al. The caregiver reaction assessment (CRA) for caregivers to persons with chronic physical and mental impairments. Res Nurs Health. 1992;15:271–283. doi: 10.1002/nur.4770150406. [DOI] [PubMed] [Google Scholar]
- 40.Luttik ML, Jaarsma T, Tijssen JG, et al. The objective burden in partners of heart failure patients; development and initial validation of the Dutch Objective Burden Inventory. Eur J Cardiovasc Nurs. 2008;7:3–9. doi: 10.1016/j.ejcnurse.2007.02.005. [DOI] [PubMed] [Google Scholar]