Figure 4.
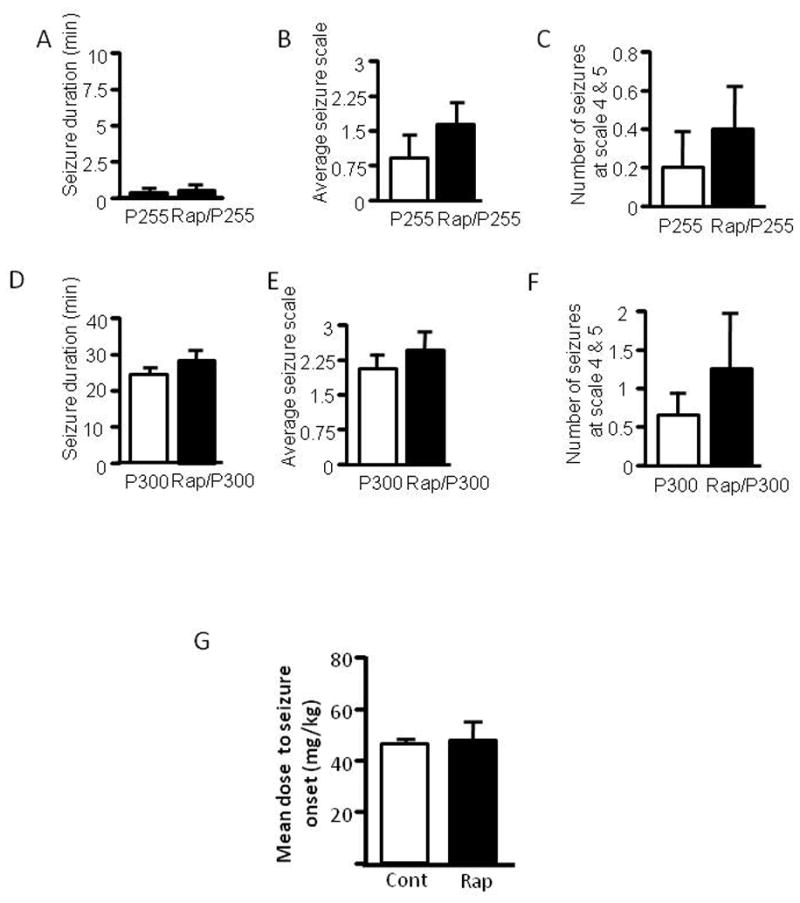
Rapamycin does not change seizure susceptibility to pilocarpine and PTZ in adult rats. Adult rats were treated with rapamycin (labeled as Rap) or vehicle for three days prior to induction of seizures by pilocarpine at 255 mg/kg (labeled as P255) (A–C) or 300 mg/kg (labeled as P300) (D–F). Behavioral seizures induced by pilocarpine at 255mg/kg are presented as average seizure duration (min) (pilocarpine: 0.36 3 ± 0.211vs rapamycin/pilocarpine: 0.507 ± 0.244) (A), average seizure scale (pilocarpine: 0.950 ± 0.477 vs. rapamycin/pilocarpine: 1.65 ± 0.504) (B) and average number of stage 4 and 5 seizures (pilocarpine: 0.200 ± 0.200 vs. rapamycin/pilocarpine: 0.400 ± 0.245) (C). Behavioral seizures induced by pilocarpine at 300 mg/kg are presented as average seizure duration (min) (pilocarpine: 23.18 ± 2.75 vs. rapamycin/pilocarpine: 26.81 ± 6.03) (D), average seizure scale (pilocarpine: 1.87 ± 0.51 vs. rapamycin/pilocarpine: 2.29 ± 0.27) (E) and average number of stage 4 and 5 seizures (pilocarpine: 0.600 ± 0.240 vs. rapamycin/pilocarpine: 1.25 ± 0.63) (F). Adult rats were treated with rapamycin or vehicle for three days followed by PTZ. Data are presented as average minimal PTZ doses (Control: 46 ± 2.92 vs. rapamycin 48 ± 7 mg) (G). (mean ± SEM; n=6–10).
