Fig 1.
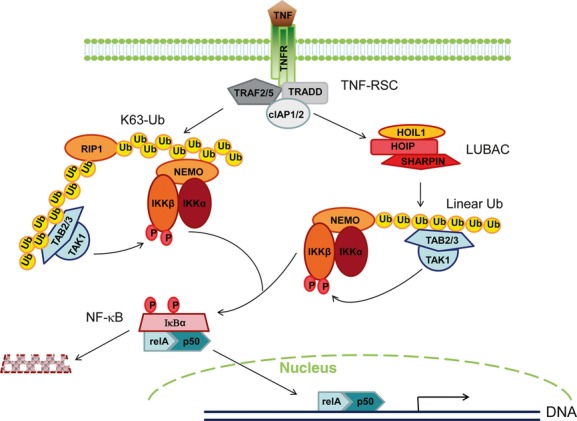
A proposed mechanistic model on how SHARPIN regulates TNF-induced NF-κB activation through linear ubiquitination-mediated mechanisms. TNF ligation induces TNFR1 trimerization and promotes the assembly of a multiprotein complex named TNF-RSC (TNF receptor signalling complex), which is composed of adaptor protein TRADD, TRAF2/5 and E3 ubiquitin ligases cIAP1/2. The signalling transduction then bifurcates into ‘TNFR-Lys63 Ub’ and ‘TNFR-linear Ub’ branches that are dependent upon different ubiquitin-dependent regulatory strategies. The first downstream ‘TNFR-Lys63 Ub’ cascade utilizes the E3 ligases cIAP1/2 to catalyse the formation of Lys63-linked ubiquitin chains to RIP1, which recruits and brings in spatial proximity the two kinase complexes TAK1-TAB2/3 and NEMO-IKKα-IKKβ. The activated kinase TAK1 then phosphorylates IKKα, which further phosphorylates IκBα to promote its proteasomal degradation. The other downstream ‘TNFR-linear Ub’ cascade employs linear ubiquitin chain-dependent mechanisms. TNF-RSC can function as a platform to attract LUBAC components, including HOIL1, HOIP and SHARPIN. LUBAC induces the formation of the linear ubiquitin chain to NEMO, which in turn recruits the kinase complex TAK1-TAB2/3. TAK1 then phosphorylates and activates the IKK complex similar to the ‘TNFR-Lys63 Ub’ pathway. Both ‘TNFR-Lys63 Ub’ and ‘TNFR-Linear Ub’ cascades converge at the IκBα-RELA-p50 complex that migrates into the nucleus for downstream gene activation after IκBα release and degradation.
