Figure 5.
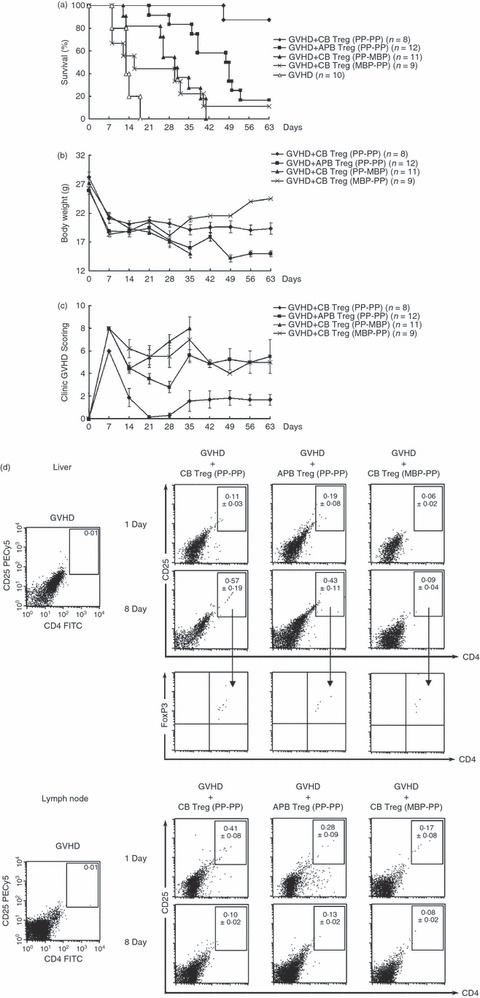
Adoptive transfer of ex vivo expanded regulatory T cells (Tregs) from cord blood (CB) and adult peripheral blood (APB) into mice with acute graft-versus-host disease (aGVHD). CB and APB Tregs were expanded ex vivo with two cycles of polyclonal stimulation: CB Treg (PP-PP) and APB Treg (PP-PP). CB Tregs were also expanded with polyclonal stimulation in the first cycle and xenogenic mouse B-cell stimulation in the second cycle or with xenogenic mouse B-cell stimulation in the first cycle and polyclonal stimulation in the second cycle: CB Treg (PP-MBP) and CB Treg (MBP-PP). In the aGVHD mouse model, 1 × 106 of the variously expanded Tregs were adoptively transferred into lethally irradiated recipient DBA/2 (H-2Kd) mice after injection with 3 × 107 bone marrow cells and 1 × 107 spleen cells from donor C57BL/6 mice (H-2Kb). The survival (a), body weight (b) and clinical GVHD score (c) were assessed. The GVHD score and body weight were determined from the surviving mice. (d) The mononuclear cells from the liver and lymph node of aGVHD mice were isolated by density gradient centrifugation using Ficoll–Hypaque on Day 1 or Day 8 after adoptive transfer of ex vivo expanded Tregs. The human molecules of CD4, CD25 and FoxP3 were determined by flow cytometry. Data represent the average percentage of human CD4+ CD25+ T cells ± standard deviation (SD) of six independent experiments.
