Fig. 3.
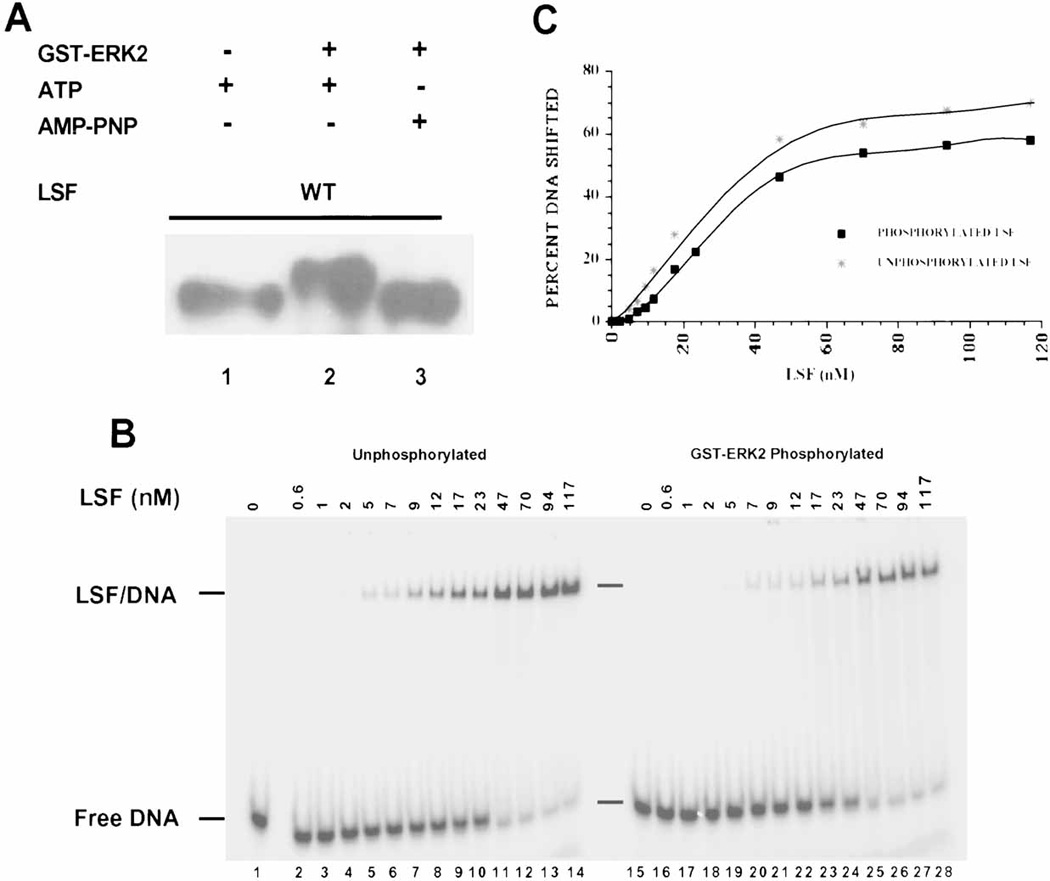
In vitro phosphorylation of LSF by ERK2 does not alter its DNA-binding activity. A: Western blot analysis of LSF phosphorylated by GST-ERK2 in vitro. Lane 1: 1 µg of His-LSF incubated only with ATP, lane 2: 1 µg of His-LSF incubated with activated GST-ERK2 and ATP, lane 3: 1 µg of His-LSF incubated with activated GST-ERK2 and AMP-PNP. The separated products of the kinase reactions were probed with α-LSFpep1-1 antibody. B: Increasing amounts of either unphosphorylated (lanes 1–14) or GST-ERK2 phosphorylated (lanes 15–28) His-LSF were analyzed by EMSA. Positions of free DNA and LSF/DNA complexes and concentrations of LSF monomer in nM are indicated. C: The averaged data of two experiments, including that shown in panel B, are plotted as a function of concentration of LSF monomer in the reaction. DNA binding is expressed as the percentage of DNA that formed a complex with LSF in the reaction. (Note: LSF binds DNA as a homotetramer.)
