Abstract
Human aging is associated with the deterioration of long-lived proteins. Gradual cumulative modifications to the life-long proteins of the lens may ultimately be responsible for the pronounced alterations to the optical and physical properties that characterize lenses from older people. γS crystallin, a major human lens protein, is known to undergo several age-dependent changes. Using proteomic techniques, a site of deamidation involving glutamine 92 has been characterized and its time course established. The proportion of deamidation increased from birth to teen-age years and then plateaud. Deamidation at this site increased again in the eighth decade of life. There was no significant difference in the extent of deamidation between cataract and age-matched normal lenses. Gln92 is located in the linker region between the two domains, and the introduction of a negative charge at this site may alter the interaction between the two regions of the protein. Gln170, which is located in another unstructured part of γS crystallin, showed a similar deamidation profile to that of Gln92. As the other Gln residues in β-sheet regions of γS crystallin appear to remain as amides, modification of Gln92 and Gln170 thus conforms to a pattern whereby deamidation is localized to the unstructured regions of long-lived proteins.
Keywords: deamidation, glutamine, age-related cataract, human lens, racemization
Introduction
One aspect of human aging involves the degradation of long-lived proteins. This takes place at a number of sites and organs in the body.1–6 Because of the presence of multiple cell types, cell complexity, low protein copy numbers, and the insolubility of long-lived proteins at some sites, it is neither straightforward to characterize the major sites of modification nor to obtain data on the time course of each modification.
These limitations are obviated in the human lens as the major structural proteins, the crystallins, are abundant and consist of fewer than 10 major polypeptides. There is also no turnover of proteins after they are packaged into a single type of cell: fiber cells.7 In addition, because lens proteins belong to more than one family, it may be possible to derive lessons on age-related polypeptide post-translational modification (PTM) that apply more widely in the body.
Maintenance of the structure of one crystallin, γS crystallin, appears to be crucial for long-term transparency of the lens, as two conservative point mutations (Gly-Val)8 and (Val-Met)9 have been shown to be responsible for human hereditary cataract. Deamidation of γ-crystallins can significantly affect protein structure,10 and therefore, it is important to characterize the sites and rates of significant deamidation in the human lens. Unfortunately, there is controversy in the literature concerning the sites and extent of deamidation in γS crystallin. With regard to Gln92, a residue located in the crucial linker region between the two domains, some authors have reported no deamidation,11,12 whereas others have found significant deamidation13,14 in older lenses.
In an effort to obtain information on the predominant types, and time courses, of modifications to proteins that turnover slowly, and are therefore submerged in physiological solution for decades, we have undertaken a program of research to determine crystallin modifications from human lenses of different ages.
In this study, we characterized a site of glutamine (Gln) deamidation in γS crystallin and monitored its rate of conversion to Glu92 as a function of time and compared this with deamidation of Gln170 and Glns in β-sheet regions of the protein.
Results
With age, both Gln and asparagine (Asn) residues can undergo deamidation.13–19 When a peptide or protein is deamidated, its molecular weight increases by 0.98 Da at each site.20 In this project, we first analyzed a tryptic peptide, AVHLPSGGQYK, derived from γS crystallin that appeared on the basis of LC/MS investigation, to show age-dependent deamidation. As the triply charged molecular ion for the original Gln-containing tryptic peptide has a mass of 386.2, the deamidated form should have a mass of 386.5. These two versions had different LC retention times, with the Gln92-containing version eluting first (∼48 min), followed by the deamidated form (50–51.5 min).
To confirm the sequences of the two peptides, tandem mass spectrometry (MS/MS) was carried out, as there should be an increase in mass for some of the fragment ions if the peptide is indeed deamidated.21 Two observations were made from the MS/MS spectra (Fig. 1). First, the experimental masses for the y-ions for the Gln-containing version AVHLPSGGQYK matched closely the theoretical values (Fig. 1). In contrast, there was an increase in mass (1 Da) for y5 (553.282), y6 (640.307), and y7 (737.354) fragments for the deamidated peptide AVHLPSGGEYK. Second, some b-ion fragments for the deamidated version also had a mass of 1 Da higher than the amidated form e.g. b8 (719.336), which confirmed the site of deamidation at Gln92. Having established by MS/MS that the Gln-containing, and deamidated, versions of the peptide were separated by LC, the proportion of each in lens protein digests could be calculated as a function of age.
Figure 1.
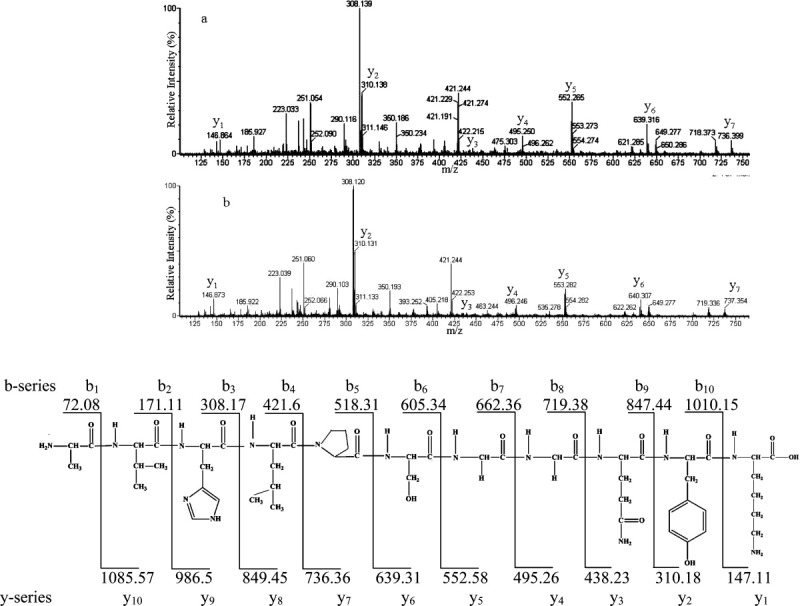
MS/MS spectra of (a) AVHLPSGGQYK (m/z = 386.2) and (b) the corresponding deamidated form of the peptide (m/z = 386.5). Spectra were obtained from separated HPLC peaks of a tryptic digest from an 88-year-old human lens. (c) The theoretical y- and b-ion fragments of AVHLPSGGQYK from γS crystallin. Masses were generated using the molecular weight calculator (http://ncrr.pnl.gov/software/).
The time course of deamidation of Gln92 in γS crystallin, as a function of age, is depicted in Figure 2. There was a linear increase in the proportion of the deamidated form with age, till the mid-teens, after which there was no further increase for the next 50 years. Following that there was a small increase from about age 70 to ∼ 30% deamidation (Fig. 2).
Figure 2.
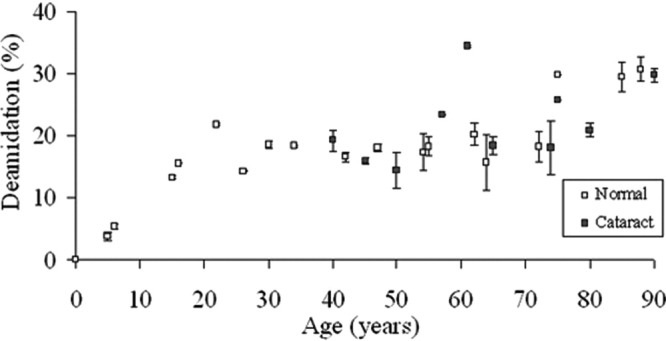
Deamidation of Gln92 in γS crystallin as a function of age. The degree of deamidation of AVHLPSGGQYK is shown for normal and cataract lens proteins. Normal: y = − 026x + 94.1, R2 = 0.725, two-sided p < 0.0001. Cataract: y = − 0.182x + 89.6, R2 = 0.213, two-sided p = 0.179). Mann-Whitney U-test, two-sided p = 0.671.
The time course of deamidation of another Gln, Gln170, in γS crystallin, as a function of age, is depicted in Figure 3. As can be seen from the crystal structure (Fig. 4), Gln170 is located in another unstructured region of γS crystallin. Up until the age of 60 years, it showed a very similar profile of deamidation with time to that of Gln92. A major difference was in the extent of deamidation, which was approximately an order of magnitude lower than that for Gln92.
Figure 3.
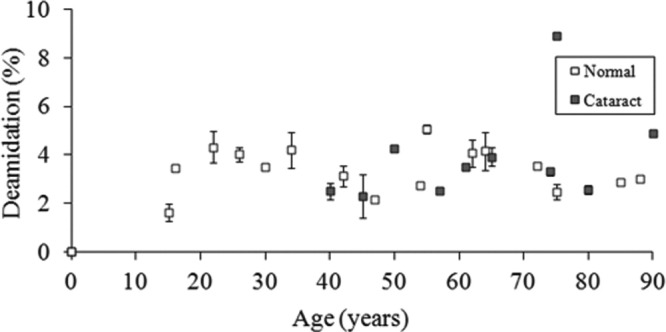
Deamidation of Gln170 in γS crystallin as a function of age. The degree of deamidation of KPIDWGAASPAVQSFR is shown for normal and cataract lens proteins. Normal: y = − 0.22x + 2.07, R2 = 0.189, two-sided p = 0.0716. Cataract: y = 0.0527x + 0.514, R2 = 0.187, two-sided p = 0.212). Mann-Whitney U-test, two-sided p = 0.853.
Figure 4.
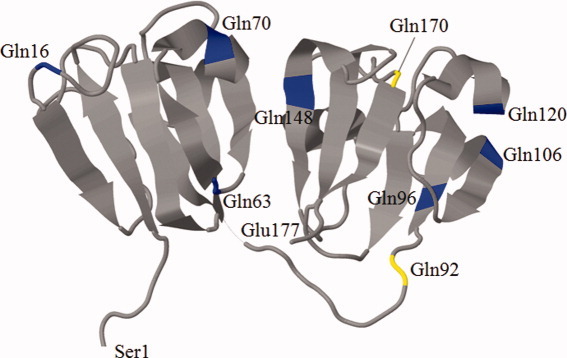
A three-dimensional representative structure of murine γS crystallin obtained from the Protein Data Bank (PDB; http://www.rcsb.org/pdb/home/home.do) showing all possible Gln residues in γS crystallin (blue) with Gln residues 92 and 170 (yellow). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
A group of cataract lenses was treated in the same manner as the normal lenses, and the results are also presented in Figures 2 and 3. It is important to note that age-related cataract lenses are available only after age ∼ 40. There were no significant differences in the deamidation values for either Gln92 or Gln170 when compared with proteins from age-matched normal lenses.
Discussion
Deamidation of Asn and Gln residues takes place with age, in which the side chain amides are converted to aspartic acid and glutamic acid, respectively, and this appears to be a common PTM of long-lived proteins.20,22 In the human lens, deamidation is implicated in protein denaturation, as deamidated residues are found more commonly in insoluble crystallins.15,23
One rationale of this study was to resolve a conflict in the literature regarding deamidation of Gln92. Two studies suggested that there was no deamidation of this residue11,12; however, we obtained clear mass spectral evidence that there is significant deamidation at this site (Fig. 1). A probable reason for the difference in the two datasets is that the earlier studies that found no deamidation were based solely on HPLC elution times in the absence of mass spectral data.11,12 Apparently, this method is subject to interference by coeluting species and is not sufficiently sensitive to detect deamidation.
There are a total of nine Gln residues in γS crystallin, yet few13 appear to undergo significant deamidation. Gln92 is located in the linker region that joins the two domains (Fig. 4). One possible reason for preferential deamidation at Gln92 is that it may be more accessible to solvent and/or is able to adopt a greater range of structures than residues located in the two domains. Conformational flexibility seems to allow a greater degree of PTM. For example, Srivastava and Srivastava24 have shown that cleavage at the adjacent GG sequence occurs in older lenses and other data (unpublished) shows that the Ser residue on the other side of the GG sequence also undergoes spontaneous cleavage.
Deamidation in proteins is not only affected by tertiary structure but is also influenced by the sequences of amino acid residues adjacent to the Asn or Gln.20,25,26 When small residues such as glycine and alanine are adjacent to amide-containing side chains, they tend to promote deamidation.27,28 Therefore, the Gly-Gly sequence in γS crystallin immediately preceding Gln92 should also act to facilitate deamidation.
As deamidation results in the formation of a carboxylate anion, this can alter ionic interactions and protein conformation. Analysis using Jmol software29 revealed that Gln92 is in close proximity to Glu133 (8.6 Å), Glu112 (10 Å), Asp113 (12.7 Å), and Glu109 (13 Å). Thus, the formation of a Glu residue at position 92 might be expected to cause significant charge–charge repulsion and potentially to lead to a more open, unfolded tertiary structure. This addition of a negative charge could interrupt the short-range interactions of human γS crystallin that have been previously observed.30 Recent data show that overall deamidation is more prevalent in insoluble proteins than soluble proteins from the same lens,15 supporting the proposal that deamidation can contribute to denaturation and aggregation.10,31–33
In the case of Gln92 and Gln170, the degree of deamidation did not differ significantly between cataract and age-matched normal lenses, which is in agreement with previous data.13 This may suggest that these particular modifications may contribute to age-related changes to the physical properties of the human lens, but not to cataract formation. On the other hand, other amides in γS crystallin such as Asn14, Asn76, and Gln16 do show distinct differences in the extent of deamidation between cataract and age-matched normal lenses and it could therefore be speculated that these may be causative for cataract.13 In relation to this point, it should be emphasized that small changes to γ-crystallins, even those that do not cause major changes to the structure of the proteins,34,35 may be sufficient to induce cataract.
Previous results for γS crystallin had indicated that by the age of 60 years, the degree of deamidation for Gln92 was ∼ 20%,13 which is in agreement with the data in this study and also corresponds with data from just three lenses reported by Hanson et al.14 In this study, we mapped the time course of deamidation and found that the percentage of deamidation increased during childhood and early teens, then remained constant at ∼ 20% for five decades (Fig. 2). There was a further increase to ∼ 30% in the mid-70s. The reason for this unusual time course is not known. It is possible that one reason for this pattern is that other modifications are taking place at separate regions of the same protein. These may affect protein structure and could therefore influence the extent of deamidation. For example, modifications to γ-crystallins include racemization of Asp/Asn11,14,36 and truncation.24 Based on this time course, it does not therefore appear feasible to calculate protein age simply on the basis of the degree of deamidation.
These data illustrate another feature that “not all Glns are created equal.” There are nine Gln residues in γS crystallin, and our results clearly show that Gln92 is deamidated, as is Gln170. Both of these are in unstructured regions of the protein (Fig. 4), as is Gln16, which also appears to be deamidated13; however, this was not the focus of this investigation. By contrast, the other Glns are located in β-sheet regions of the protein and are either not deamidated or modified to a degree that was below the level of detection. The one possible exception to this rule is Gln120, which was reported to be deamidated in a previous publication.13 A Gln in the linker region of γC crystallin is also deamidated with age.13 In this sense, the Gln data are in accord with Asn deamidation in aged proteins. For example, in γS crystallin, Asn76 in the linker region is the most highly deamidated Asn residue.37
Taken together, a hypothesis can be constructed in which deamidation in aged proteins is determined largely by secondary structure, rather than other factors such as nearby residues; although these may influence the degree to which individual Asn and Gln amino acids are deamidated. The importance of secondary structure of proteins in determining deamidation was proposed by Clarke22 and is supported by data on succinimide hydrolysis in proteins.38
As noted above, a picture is emerging from an examination of several proteins as a function of age, both in the lens and outside of the eye. Flexible regions appear to be more prone to age-related modifications such as deamidation and racemization than structured regions.22,39 In addition, certain amino acid residues, such as Asp, Asn, and Ser, appear to be particularly susceptible to age-related modifications. Understanding the main factors that are responsible for the major PTMs of long-lived proteins may allow us in the future to predict those polypeptides that are most susceptible to age-related PTM and also the regions of these proteins that are most at risk. Such information will assist in characterizing PTMs in proteins such as the nuclear pore proteins that are known to undergo major age-related changes that affect function,40 but as yet are unexplored in terms of the significant sites of age-related modification.
Conclusion
Structural proteins, such as γS crystallin, are present in the human lens for many decades. Two sites of deamidation, one involving Gln92 in the linker region of γS crystallin and the other Gln170, were characterized. The degree of deamidation for each was found to increase from birth to teen-age years and then to remain constant for four decades. Both sites that deamidated are in unstructured regions of the protein. There was no significant difference in the deamidation of Gln92 or Gln170 between cataract and age-matched normal lenses.
Methods and Materials
Extraction and tryptic digestion of lens proteins
Normal human lenses were obtained from the Sydney Eye Bank, with ethical approval from the University of Sydney and fetal lenses from the Endocrinology Department, Prince of Wales Hospital, Randwick, NSW, Australia. Cataractous lenses were obtained from the K.T. Seth Eye Hospital, Rajkot, Gujarat, India. Proteins were extracted using a previous method.41 Total proteins from the lens nucleus were extracted following a previous protocol.37
Liquid chromatography mass spectrometry
Tandem mass spectra were acquired using a Waters/Micromass quadruple time-of-flight (QTOF) Ultima mass spectrometer with a nanospray source (Manchester, UK). Tandem mass spectra were acquired using a Waters/Micromass QTOF Ultima mass spectrometer with a nanospray source (Manchester, UK). Peptides were separated by 1D LC using a nanoCap-LC auto-sampler system (Waters, Milford, MA) as described for αA crystallin.37
Data analysis
The triply charged ions for the γS crystallin-derived tryptic peptides for both amidated [m/z = 386.2 (Gln92) and 577.3 (Gln170)] and deamidated [m/z = 386.5 (Gln92) and 577.6 (Gln170)] were extracted. Their intensities from the extracted ion chromatogram (XIC) were determined using MassLynx software. Peak areas of specific peptides were calculated using a mean smoothing method [number of smooths: 2, window size (scans): ±3]. The MS/MS spectrum of each peptide was matched to the XIC, ensuring that the peak areas used corresponded to that of the matched peptide and not to an isobaric peptide. The percentage of deamidation was calculated by using the following formula: [deamidated/(amidated + deamidated)] × 100. A simple linear regression analysis and Mann-Whitney U-test were performed using a previous method.41
References
- 1.Ritz-Timme S, Collins MJ. Racemization of aspartic acid in human proteins. Ageing Res Rev. 2002;1:43–59. doi: 10.1016/s0047-6374(01)00363-3. [DOI] [PubMed] [Google Scholar]
- 2.Truscott RJW. Are ancient proteins responsible for the age-related decline in health and fitness? Rejuvenation Res. 2010;13:83–89. doi: 10.1089/rej.2009.0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro S, Endicott S, Province M, Pierce J, Campbell E. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of d-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87:1828–1834. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powell J, Vine N, Crossman M. On the accumulation of d-aspartate in elastin and other proteins of the ageing aorta. Atherosclerosis. 1992;97:201–208. doi: 10.1016/0021-9150(92)90132-z. [DOI] [PubMed] [Google Scholar]
- 5.Shapira R, Wilkinson KD, Shapira G. Racemization of individual aspartate residues in human myelin basic protein. J Neurochem. 1988;50:649–654. doi: 10.1111/j.1471-4159.1988.tb02960.x. [DOI] [PubMed] [Google Scholar]
- 6.Helfman PM, Bada JL. Aspartic acid racemization in tooth enamel from living humans. Proc Natl Acad Sci USA. 1975;72:2891–2894. doi: 10.1073/pnas.72.8.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C, Heinemeier J. Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS One. 2008;1:1529–1531. doi: 10.1371/journal.pone.0001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun H, Ma Z, Li Y, Liu B, Li Z, Ding X, Gao Y, Ma W, Tang X, Li X, Shen Y. γ-S crystallin gene (CRYGS) mutation causes dominant progressive cortical cataract in humans. J Med Genet. 2005;42:706–710. doi: 10.1136/jmg.2004.028274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanita V, Singh JR, Singh D, Varon R, Sperling K. Novel mutation in the γ-S crystallin gene causing autosomal dominant cataract. Mol Vis. 2009;15:476–481. [PMC free article] [PubMed] [Google Scholar]
- 10.Flaugh SL, Mills IA, King J. Glutamine deamidation destabilizes human γD-crystallin and lowers the kinetic barrier to unfolding. J Biol Chem. 2006;281:30782–30793. doi: 10.1074/jbc.M603882200. [DOI] [PubMed] [Google Scholar]
- 11.Takemoto L. Deamidation of Asn-143 of γS crystallin from protein aggregates of the human lens. Curr Eye Res. 2001;22:148–153. doi: 10.1076/ceyr.22.2.148.5524. [DOI] [PubMed] [Google Scholar]
- 12.Takemoto L, Boyle D. Specific glutamine and asparagine residues of γ-S crystallin are resistant to in vivo deamidation. J Biol Chem. 2000;275:26109–26112. doi: 10.1074/jbc.M002809200. [DOI] [PubMed] [Google Scholar]
- 13.Hains P, Truscott RJ. Age-dependent deamidation of life-long proteins in the human lens. Invest Opthalmol Vis Sci. 2010;51:3107–3114. doi: 10.1167/iovs.09-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanson SRA, Smith DL, Smith JB. Deamidation and disulfide bonding in human lens [γ]-crystallins. Exp Eye Res. 1998;67:301–312. doi: 10.1006/exer.1998.0530. [DOI] [PubMed] [Google Scholar]
- 15.Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, Pevzner PA, David LL. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J Proteome Res. 2006;5:2554–2566. doi: 10.1021/pr050473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Z, Hanson SRA, Lampi KJ, David LL, Smith DL, Smith JB. Age-related changes in human lens crystallins identified by HPLC and mass spectrometry. Exp Eye Res. 1998;67:21–30. doi: 10.1006/exer.1998.0482. [DOI] [PubMed] [Google Scholar]
- 17.Groenen PJTA, van Dongen MJP, Voorter CEM, Bloemendal H, de Jong WW. Age-dependent deamidation of αB-crystallin. FEBS Lett. 1993;322:69–72. doi: 10.1016/0014-5793(93)81113-e. [DOI] [PubMed] [Google Scholar]
- 18.Lampi KJ, Ma Z, Hanson SRA, Azuma M, Shih M, Shearer TR, Smith DL, Smith JB, David LL. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Exp Eye Res. 1998;67:31–43. doi: 10.1006/exer.1998.0481. [DOI] [PubMed] [Google Scholar]
- 19.Lindner H, Helliger W. Age-dependent deamidation of asparagine residues in proteins. Exp Gerontol. 2001;36:1551–1563. doi: 10.1016/s0531-5565(01)00140-1. [DOI] [PubMed] [Google Scholar]
- 20.Robinson NE, Robinson AB. Molecular clocks: deamidation of asparaginyl and glutaminyl residues in peptides and proteins. Cave Junction, OR: Althouse Press; 2004. [Google Scholar]
- 21.Lapko VN, Purkiss AG, Smith DL, Smith JB. Deamidation in human γS-crystallin from cataractous lenses is influenced by surface exposure. Biochemistry. 2002;41:8638–8648. doi: 10.1021/bi015924t. [DOI] [PubMed] [Google Scholar]
- 22.Clarke S. Propensity for spontaneous succinimide formation from aspartyl and asparaginyl residues in cellular proteins. Int J Pept Prot Res. 1987;30:808–821. doi: 10.1111/j.1399-3011.1987.tb03390.x. [DOI] [PubMed] [Google Scholar]
- 23.Hanson SR, Hasan A, Smith DL, Smith JB. The major in vivo modifications of the human water-insoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation and backbone cleavage. Exp Eye Res. 2000;71:195. doi: 10.1006/exer.2000.0868. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava OP, Srivastava K. Degradation of γD- and γS-crystallins in human lenses. Biochem Biophys Res Commun. 1998;253:288–294. doi: 10.1006/bbrc.1998.9728. [DOI] [PubMed] [Google Scholar]
- 25.Robinson AB, Robinson LR. Distribution of glutamine and asparagine residues and their near neighbors in peptides and proteins. Proc Natl Acad Sci USA. 1991;88:8880–8884. doi: 10.1073/pnas.88.20.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson NE, Robinson AB. Deamidation of human proteins. Proc Natl Acad Sci USA. 2001;98:12409–12413. doi: 10.1073/pnas.221463198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geiger T, Clarke S. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J Biol Chem. 1987;262:785–794. [PubMed] [Google Scholar]
- 28.Stephenson RC, Clarke S. Succinimide formation from aspartyl and asparaginyl peptides as a model for the spontaneous degradation of proteins. J Biol Chem. 1989;264:6164–6170. [PubMed] [Google Scholar]
- 29.Willighagen EL, Howard M. Fast and scriptable molecular graphics in web browsers without Java3D. Nat Proc. 2007 doi: 10.1038/npre.2007.50.1. [Google Scholar]
- 30.Purkiss AG, Bateman OA, Goodfellow JM, Lubsen NH, Slingsby C. The X-ray crystal structure of human γS-crystallin C-terminal domain. J Biol Chem. 2002;277:4199–4205. doi: 10.1074/jbc.M110083200. [DOI] [PubMed] [Google Scholar]
- 31.Takata T, Oxford JT, Demeler B, Lampi KJ. Deamidation destabilizes and triggers aggregation of a lens proteins, A3 crystallin. Protein Sci. 2008;17:1565–1575. doi: 10.1110/ps.035410.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta R, Srivastava OP. Effect of deamidation of asparagine 146 on functional and structural properties of human lens αB-crystallin. Invest Ophthal Vis Sci. 2004;45:206–214. doi: 10.1167/iovs.03-0720. [DOI] [PubMed] [Google Scholar]
- 33.Lampi KJ, Oxford JT, Bachinger HP, Shearer TR, David LL, Kapfer DM. Deamidation of human βB1 alters the elongated structure of the dimer. Exp Eye Res. 2001;72:279–288. doi: 10.1006/exer.2000.0950. [DOI] [PubMed] [Google Scholar]
- 34.Pande A, Pande J, Asherie N, Lomakin A, Ogun O, King JA, Lubsen NH, Walton D, Benedek GB. Molecular basis of a progressive juvenile-onset hereditary cataract. Proc Natl Acad Sci USA. 2000;97:1993–1998. doi: 10.1073/pnas.040554397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basak A, Bateman O, Slingsby C, Pande A, Asherie N, Ogun O, Benedek GB, Pande J. High-resolution X-ray crystal structures of human γD crystallin (1.25 Å) and the R58H mutant (1.15 Å) associated with aculeiform cataract. J Mol Biol. 2003;328:1137–1147. doi: 10.1016/s0022-2836(03)00375-9. [DOI] [PubMed] [Google Scholar]
- 36.Masters PM, Bada JL, Zigler JS. Aspartic acid racemization in heavy molecular weight crystallins and water insoluble protein from normal human lenses and cataracts. Proc Natl Acad Sci USA. 1978;75:1204–1208. doi: 10.1073/pnas.75.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hooi MYS, Raftery MJ, Truscott RJW. Racemisation of two proteins over our lifespan. Deamidation of asparagine 76 in γS crystallin is greater in cataract than in normal lenses across the age range. Invest Ophthal Vis Sci. doi: 10.1167/iovs.11-9085. doi: 10.1167/iovs.11-9085. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Athmer L, Kindrachuk J, Georges F, Napper S. The influence of protein structure on the products emerging from succinimide hydrolysis. J Biol Chem. 2002;277:30502–30507. doi: 10.1074/jbc.M205314200. [DOI] [PubMed] [Google Scholar]
- 39.Radkiewicz JL, Zipse H, Clarke S, Houk KN. Neighboring side chain effects on asparaginyl and aspartyl degradation: an ab initio study of the relationship between peptide conformation and backbone NH acidity. J Am Chem Soc. 2001;123:3499–3506. doi: 10.1021/ja0026814. [DOI] [PubMed] [Google Scholar]
- 40.D'Angelo MA, Raices M, Panowski SH, Hetzer MW. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136:284–295. doi: 10.1016/j.cell.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hooi M, Truscott R. Racemisation and human cataract. d-Ser, d-Asp/Asn and d-Thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses. AGE. 2011;33:131–141. doi: 10.1007/s11357-010-9171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


