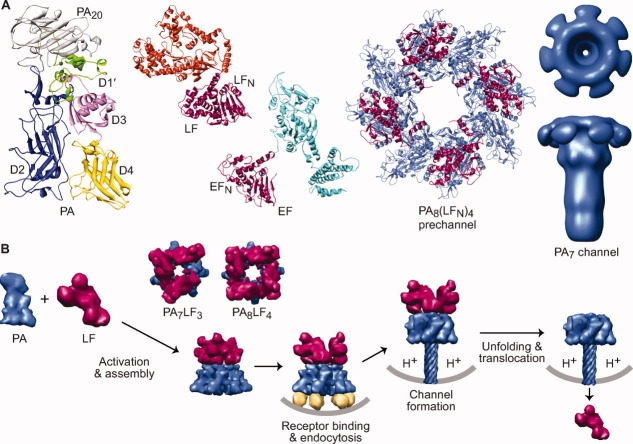
Figure 3.
An overview of the anthrax toxin protein translocation system. (A) Components of anthrax toxin (left to right). Ribbons depiction of PA (3TEW35) colored by domain: PA20 (gray), D1′ (green), Ca2+ ions (dark green), D2 (blue), D3 (magenta), and D4 (yellow). The enzymes, LF (1J7N36) and EF (1YOV37); their amino-terminal PA binding domains (LFN and EFN, respectively) are colored red-violet and their catalytic domains colored orange and cyan, respectively. A representative PA prechannel complex, PA8(LFN)4, (3KWV38); the PA oligomer and LFN colored denim and red-violet, respectively. Axial view (above) and sideview (below) of a three-dimensional EM reconstruction of the PA7 channel39 (colored denim) (Prof. Mark Fisher graciously provided the EM density map). (B) Anthrax toxin assembly and transport. PA (denim) is proteolytically nicked and assembles with LF (red-violet) and forms PA8LF4 and PA7LF3 prechannel complexes40, 41 (based on 3KWV38 and 1TZO,42 respectively). Prechannel complexes bind cellular receptors (gold; 1T6B43 and 1TZN44) triggering endocytosis; acidic pH conditions in the endosome induce PA to form a transmembrane channel39, 45, 46 (atomic model 1V3647); the pH gradient that develops across the endosomal membrane destabilizes LF,48 drives LF unfolding22, 49 and translocation21, 22 through the PA channel.