Figure 5.
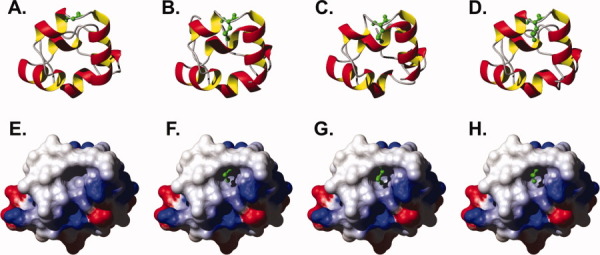
(A–D) Ribbon diagram with residue 61 represented as ball and stick for (A) HP67 L61G, 3NKJ; (B) wild-type HP67, 2RJX chain A; (C) wild-type HP67, 2RJX chain B; (D) wild-type HP67, 2RJY. (E–H) Solvent-accessible surface maps of HP67 L61G colored by electrostatic potential (computed with MolMol)19 from red (−0.3) through white (0.0), to blue (0.3). The leucine 61 side chain of wild-type villin headpiece (F, 2RJX chain A; G 2RJX chain B; H wild-type HP67, 2RJY), appropriately aligned (all atom via Topofit algorithm)20, 21 to HP67 L61G, are drawn to demonstrate that the leucine side chain fills the vacant solvent exposed hydrophobic pocket of HP67 L61G.
