Abstract
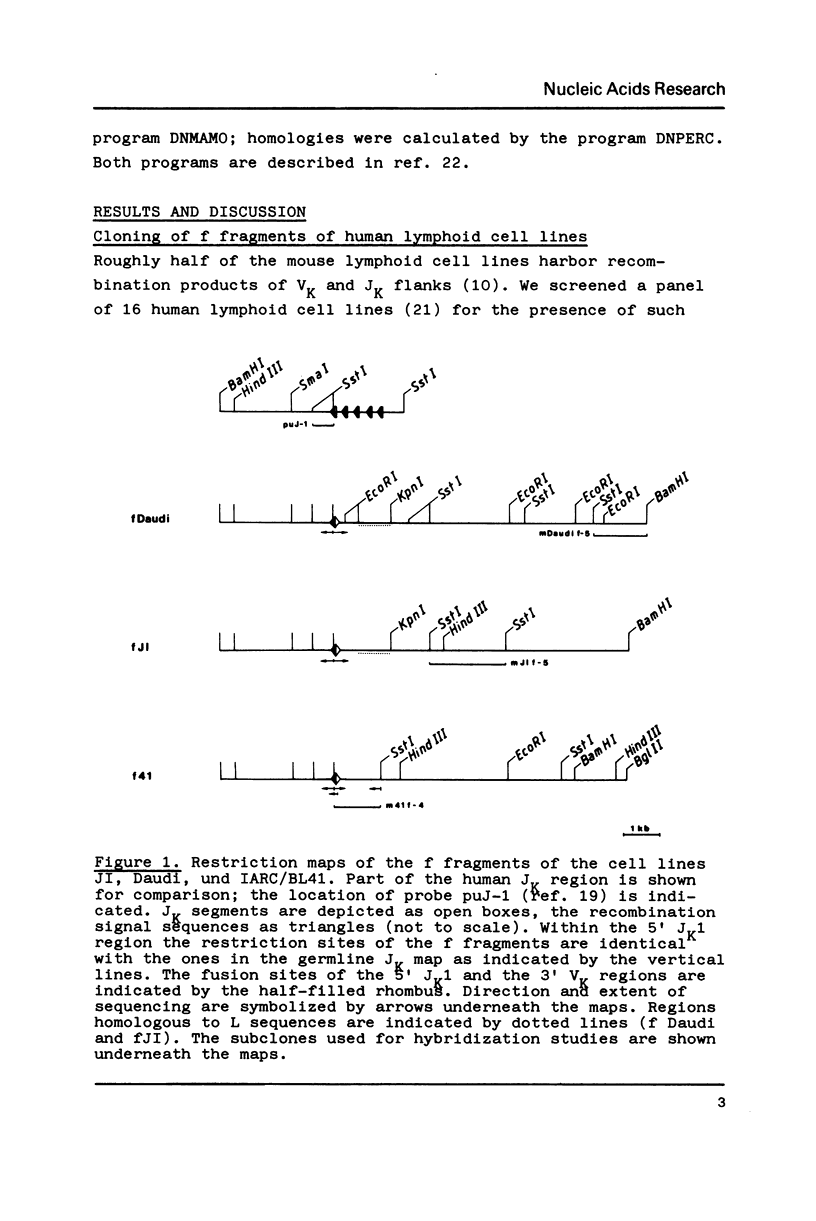
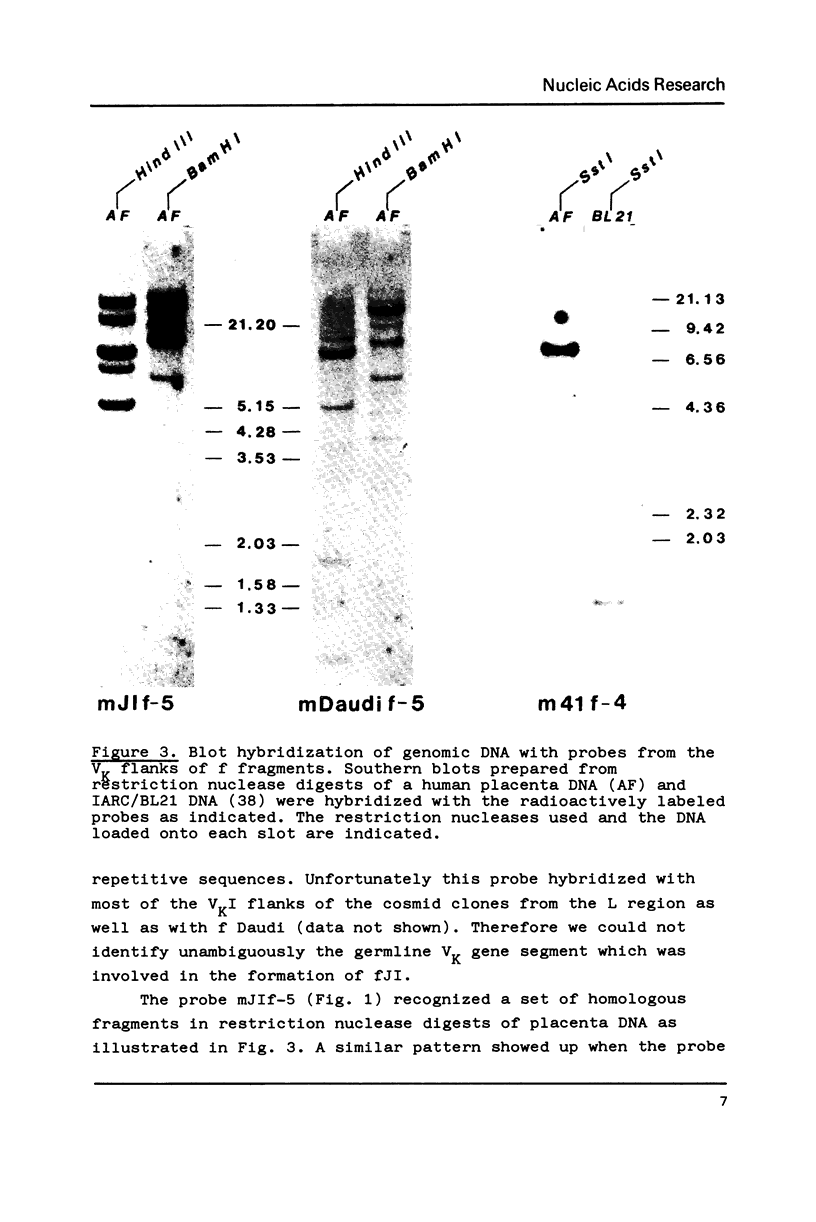
The recombination process that joins a VK to a JK segment of an immunoglobulin gene generates a second, reciprocal recombination product called f fragment. In this second product the regions flanking the VK and JK segments in the germline are joined in a head to head fashion. We now analysed f fragments in the human lymphoid cell lines Daudi, JI and IARC/BL41. All three f fragments contain JK1 flanks; the VK derived moiety of f Daudi and f41 could be traced back to known germline VK genes. There is a precise head to head joining of the heptanucleotide signal sequences in f Daudi and fJI while in f41 six nucleotides are present between the signal sequences. In contrast to the VK-JK recombination products, the f fragments were found to lack somatic mutations. The structures of the f fragments are discussed in the context of the VK-JK rearrangement mechanism.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Chen K. C., Smith S. D., Rabbitts T. H. Fusion of an immunoglobulin variable gene and a T cell receptor constant gene in the chromosome 14 inversion associated with T cell tumors. Cell. 1985 Dec;43(3 Pt 2):705–713. doi: 10.1016/0092-8674(85)90243-0. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Rabbitts T. H. Evolution of immunoglobulin V genes: evidence indicating that recently duplicated human V kappa sequences have diverged by gene conversion. Cell. 1983 Jan;32(1):181–189. doi: 10.1016/0092-8674(83)90508-1. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Rabbitts T. H. Human immunoglobulin variable region genes--DNA sequences of two V kappa genes and a pseudogene. Nature. 1980 Dec 25;288(5792):730–733. doi: 10.1038/288730a0. [DOI] [PubMed] [Google Scholar]
- Berek C., Griffiths G. M., Milstein C. Molecular events during maturation of the immune response to oxazolone. Nature. 1985 Aug 1;316(6027):412–418. doi: 10.1038/316412a0. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkamm G. W., Kaduk B., Kachel G., Schneider U., Fresen K. O., Schwanitz G., Hermanek P. Epstein-Barr virus-positive Burkitt's lymphoma in a German woman during pregnancy. Blut. 1980 Mar;40(3):167–177. doi: 10.1007/BF01008574. [DOI] [PubMed] [Google Scholar]
- Denny C. T., Hollis G. F., Hecht F., Morgan R., Link M. P., Smith S. D., Kirsch I. R. Common mechanism of chromosome inversion in B- and T-cell tumors: relevance to lymphoid development. Science. 1986 Oct 10;234(4773):197–200. doi: 10.1126/science.3092355. [DOI] [PubMed] [Google Scholar]
- Denny C. T., Yoshikai Y., Mak T. W., Smith S. D., Hollis G. F., Kirsch I. R. A chromosome 14 inversion in a T-cell lymphoma is caused by site-specific recombination between immunoglobulin and T-cell receptor loci. Nature. 1986 Apr 10;320(6062):549–551. doi: 10.1038/320549a0. [DOI] [PubMed] [Google Scholar]
- Duby A. D., Seidman J. G. Abnormal recombination products result from aberrant DNA rearrangement of the human T-cell antigen receptor beta-chain gene. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4890–4894. doi: 10.1073/pnas.83.13.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., Nishikura K., ar-Rushdi A., Finan J., Emanuel B., Lenoir G., Nowell P. C., Croce C. M. Translocation of an immunoglobulin kappa locus to a region 3' of an unrearranged c-myc oncogene enhances c-myc transcription. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7581–7585. doi: 10.1073/pnas.80.24.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feddersen R. M., Van Ness B. G. Double recombination of a single immunoglobulin kappa-chain allele: implications for the mechanism of rearrangement. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4793–4797. doi: 10.1073/pnas.82.14.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Maizel J. V., Jr, Leder P. Evolution of human immunoglobulin kappa J region genes. J Biol Chem. 1982 Feb 10;257(3):1516–1522. [PubMed] [Google Scholar]
- Honjo T., Habu S. Origin of immune diversity: genetic variation and selection. Annu Rev Biochem. 1985;54:803–830. doi: 10.1146/annurev.bi.54.070185.004103. [DOI] [PubMed] [Google Scholar]
- Höchtl J., Müller C. R., Zachau H. G. Recombined flanks of the variable and joining segments of immunoglobulin genes. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1383–1387. doi: 10.1073/pnas.79.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höchtl J., Zachau H. G. A novel type of aberrant recombination in immunoglobulin genes and its implications for V-J joining mechanism. Nature. 1983 Mar 17;302(5905):260–263. doi: 10.1038/302260a0. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Ogura T., Shimizu A., Honjo T. A joining-diversity-joining complex generated by inversion mechanism and a variable-diversity complex in the beta-chain gene of the human T-cell receptor. Nucleic Acids Res. 1986 Jun 25;14(12):4899–4909. doi: 10.1093/nar/14.12.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenichen H. R., Pech M., Lindenmaier W., Wildgruber N., Zachau H. G. Composite human VK genes and a model of their evolution. Nucleic Acids Res. 1984 Jul 11;12(13):5249–5263. doi: 10.1093/nar/12.13.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobeck H. G., Bornkamm G. W., Combriato G., Mocikat R., Pohlenz H. D., Zachau H. G. Subgroup IV of human immunoglobulin K light chains is encoded by a single germline gene. Nucleic Acids Res. 1985 Sep 25;13(18):6515–6529. doi: 10.1093/nar/13.18.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobeck H. G., Combriato G., Zachau H. G. Immunoglobulin genes of the kappa light chain type from two human lymphoid cell lines are closely related. Nucleic Acids Res. 1984 Sep 25;12(18):6995–7006. doi: 10.1093/nar/12.18.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobeck H. G., Meindl A., Combriato G., Solomon A., Zachau H. G. Human immunoglobulin kappa light chain genes of subgroups II and III. Nucleic Acids Res. 1985 Sep 25;13(18):6499–6513. doi: 10.1093/nar/13.18.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobeck H. G., Solomon A., Zachau H. G. Contribution of human V kappa II germ-line genes to light-chain diversity. Nature. 1984 May 3;309(5963):73–76. doi: 10.1038/309073a0. [DOI] [PubMed] [Google Scholar]
- Klobeck H. G., Zachau H. G. The human CK gene segment and the kappa deleting element are closely linked. Nucleic Acids Res. 1986 Jun 11;14(11):4591–4603. doi: 10.1093/nar/14.11.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir G. M., Preud'homme J. L., Bernheim A., Berger R. Correlation between immunoglobulin light chain expression and variant translocation in Burkitt's lymphoma. Nature. 1982 Jul 29;298(5873):474–476. doi: 10.1038/298474a0. [DOI] [PubMed] [Google Scholar]
- Lewis S., Gifford A., Baltimore D. DNA elements are asymmetrically joined during the site-specific recombination of kappa immunoglobulin genes. Science. 1985 May 10;228(4700):677–685. doi: 10.1126/science.3158075. [DOI] [PubMed] [Google Scholar]
- Lewis S., Gifford A., Baltimore D. Joining of V kappa to J kappa gene segments in a retroviral vector introduced into lymphoid cells. 1984 Mar 29-Apr 4Nature. 308(5958):425–428. doi: 10.1038/308425a0. [DOI] [PubMed] [Google Scholar]
- Lewis S., Rosenberg N., Alt F., Baltimore D. Continuing kappa-gene rearrangement in a cell line transformed by Abelson murine leukemia virus. Cell. 1982 Oct;30(3):807–816. doi: 10.1016/0092-8674(82)90285-9. [DOI] [PubMed] [Google Scholar]
- Malissen M., McCoy C., Blanc D., Trucy J., Devaux C., Schmitt-Verhulst A. M., Fitch F., Hood L., Malissen B. Direct evidence for chromosomal inversion during T-cell receptor beta-gene rearrangements. Nature. 1986 Jan 2;319(6048):28–33. doi: 10.1038/319028a0. [DOI] [PubMed] [Google Scholar]
- Pech M., Jaenichen H. R., Pohlenz H. D., Neumaier P. S., Klobeck H. G., Zachau H. G. Organization and evolution of a gene cluster for human immunoglobulin variable regions of the kappa type. J Mol Biol. 1984 Jun 25;176(2):189–204. doi: 10.1016/0022-2836(84)90420-0. [DOI] [PubMed] [Google Scholar]
- Pech M., Smola H., Pohlenz H. D., Straubinger B., Gerl R., Zachau H. G. A large section of the gene locus encoding human immunoglobulin variable regions of the kappa type is duplicated. J Mol Biol. 1985 Jun 5;183(3):291–299. doi: 10.1016/0022-2836(85)90001-4. [DOI] [PubMed] [Google Scholar]
- Pech M., Zachau H. G. Immunoglobulin genes of different subgroups are interdigitated within the VK locus. Nucleic Acids Res. 1984 Dec 21;12(24):9229–9236. doi: 10.1093/nar/12.24.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Selsing E., Storb U. Mapping of immunoglobulin variable region genes: relationship to the 'deletion' model of immunoglobulin gene rearrangement. Nucleic Acids Res. 1981 Nov 11;9(21):5725–5735. doi: 10.1093/nar/9.21.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsing E., Voss J., Storb U. Immunoglobulin gene 'remnant' DNA--implications for antibody gene recombination. Nucleic Acids Res. 1984 May 25;12(10):4229–4246. doi: 10.1093/nar/12.10.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer J., Kekish O., Batter D., Grenier J., Balazs I., Henderson E., Zegers B. J. Aberrant recombination events in B cell lines derived from a kappa-deficient human. Nucleic Acids Res. 1985 May 24;13(10):3495–3514. doi: 10.1093/nar/13.10.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Altenburger W., Zachau H. G. A rearranged DNA sequence possibly related to the translocation of immunoglobulin gene segments. Nucleic Acids Res. 1980 Apr 25;8(8):1709–1720. doi: 10.1093/nar/8.8.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straubinger B., Pech M., Mühlebach K., Jaenichen H. R., Bauer H. G., Zachau H. G. Molecular footprints of human immunoglobulin gene evolution: a new sequence family. Nucleic Acids Res. 1984 Jul 11;12(13):5265–5275. doi: 10.1093/nar/12.13.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Van Ness B. G., Coleclough C., Perry R. P., Weigert M. DNA between variable and joining gene segments of immunoglobulin kappa light chain is frequently retained in cells that rearrange the kappa locus. Proc Natl Acad Sci U S A. 1982 Jan;79(2):262–266. doi: 10.1073/pnas.79.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]