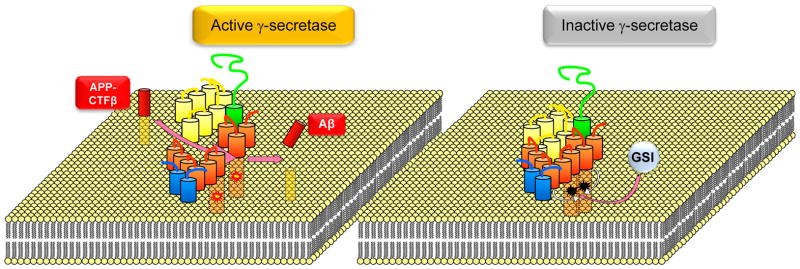
Figure 5. Mechanism of γ-secretase inhibition.
Left: γ-secretase catalyzes proteolysis of transmembrane substrates including APP-CTFβ shown here. Processing of this substrate at the active site of the enzyme which includes two aspartate residues shown as yellow-red sparks (one on PS-NTF and the other on PS-CTF) generates AICD when substrate is cleaved at the ε site and Aβ when cleaved at γ sites. Right: γ-secretase is inhibited by GSIs that bind the active site and enhance interactions between γ-secretase subunits thus locking the enzyme in a closed conformation, characteristic of inhibited enzymes (inactivated aspartates are shown in black) (Barthet et al., 2011).