FIGURE 5.
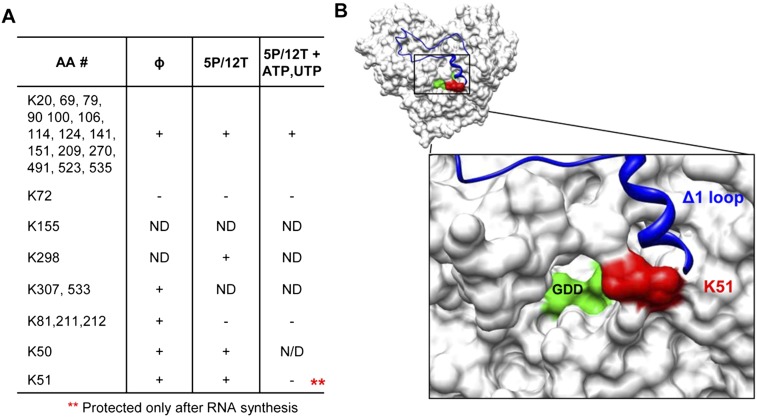
Mapping interactions in the RdRp ternary complex using an aminidation interference assay. (A) Lysines modified in the presence of S-methyl thioacetimidate (SMTA) as a function of RNA synthesis condition. Briefly, 0.3 μM 1bΔ21 was added to a 50-μL reaction containing 100 mM HEPES, 4 mM MgCl2, 12.5 mM dithiothreitol, 0.5% Triton X-100 (v/v), and the RNAs and NTPs indicated. An equal volume of 200 mM SMTA dissolved in the same buffer was added for 1 h to allow modification of all surface-exposed lysines. After exchanging the buffer with 100 mM NH4HCO3, trypsin was added and it was incubated overnight at 37°C. The peptides were then analyzed using a LTQ linear ion trap mass spectrometer (Thermo Scientific). (ND) No data were obtained for that residue. (B) Molecular models of NS5B that highlight K51, which is differentially protected during RNA synthesis. (Red) K51; (blue) the Δ1 loop; (green) the GDD active-site motif.