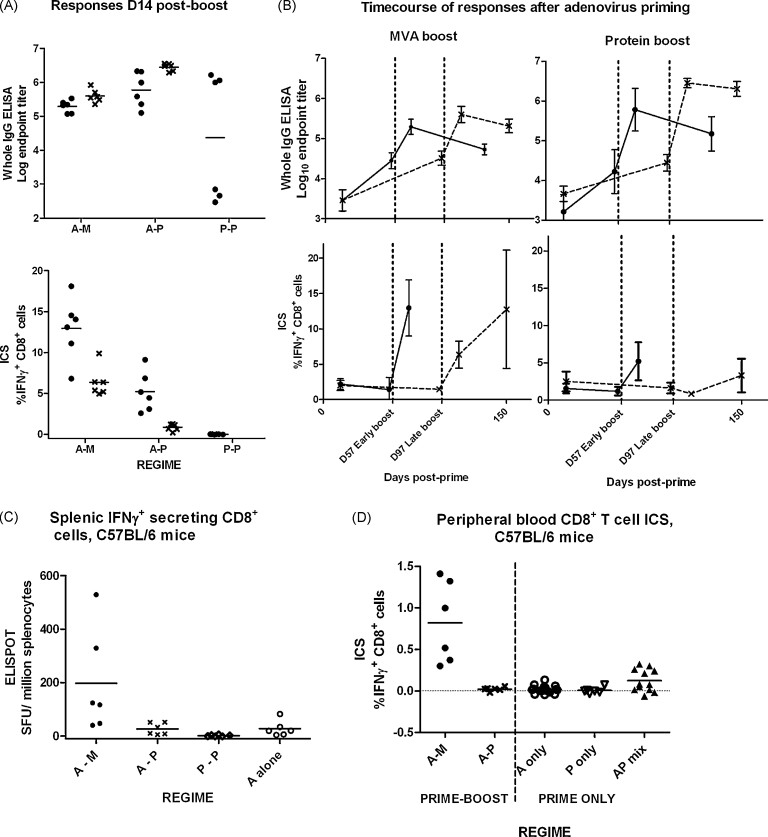
Fig. 1.
Responses induced by two-immunization regimes. In figures throughout this report, the abbreviations ‘A’, ‘M’ and ‘P’ are used in place of ‘AdCh63’, ‘MVA’ and ‘protein’, respectively. A dash is used to indicate separate sequential vaccinations whereas parentheses and a+ sign indicates mixed vaccinations–for example, ‘A–P’ indicates AdCh63 followed by protein, whereas ‘(A+P)’ indicates mixed adenovirus and protein given simultaneously at the same site. Unless otherwise stated, mice were BALB/c and doses were 1010 virus particles (vp) for AdCh63; 107 plaque forming units (pfu) for MVA; and 20 μg for protein. In this and subsequent figures, all graphs plot individual values (symbols) and group mean (line). Error bars, where present, indicate 95% confidence interval for population mean. Throughout, group size n = 6 except where otherwise specified. Panel A: Comparison of total PfMSP119 specific IgG and PfMSP133 specific CD8+ T cell responses measured by ICS 14 days post-boost in BALB/c mice. P–P 97 day dose interval not done. Graph symbols indicate dose interval: (●) for 57 days; (×) for 97 days. Panel B: Illustration of timecourses of total IgG (upper two panels) and CD8+ T cell (lower two panels) responses in adenovirus-primed BALB/c mice receiving different boosting vaccines (MVA in left two panels; protein in right-hand two panels). In each panel, the two lines illustrate responses with different dosing intervals, with graph symbols as in panel A. Panel C: C57BL/6 mice were immunized for examination of splenic IFNγ secreting CD8+ T cell response to an MSP119 epitope (peptide 215), assessed by ELISPOT for optimal sensitivity 14 days after vaccination. Doses were 108 vp AdCh63, 106 pfu MVA, and 5 μg protein. Panel D: C57BL/6 mice (n = 6–18 per group) were immunized as in Figure C, with the addition of a group receiving a single immunization with adenovirus–protein mixture. CD8+ T cell responses to peptide 215 were assessed by peripheral blood ICS 14 days after vaccination.