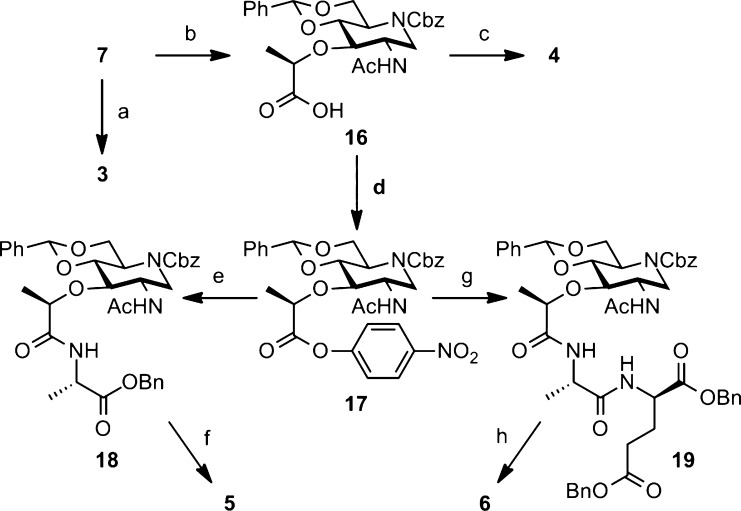
Scheme 2. Synthesis of Iminosaccharides 3–6.
Reagents and conditions: (a) H2, Pd/C, HCl, iPrOH, rt, quant.; (b) (S)-2-chloropropionic acid, NaH, THF, 60 °C, 91%; (c) H2, Pd/C, HCl, iPrOH, rt, quant.; (d) p-nitrophenyl trifluoroacetate, Py, TEA, CH2Cl2, rt, 72%; (e) l-alanine benzyl ester, TEA, THF, CH2Cl2, reflux, 85%; (f) H2, Pd/C, HCl, iPrOH, rt, quant.; (g) dibenzyl N-(l-alaninyl)-d-glutamate, TEA, THF, CH2Cl2, reflux, 90%; (h) H2, Pd/C, HCl, iPrOH, rt, quant.