Figure 2.
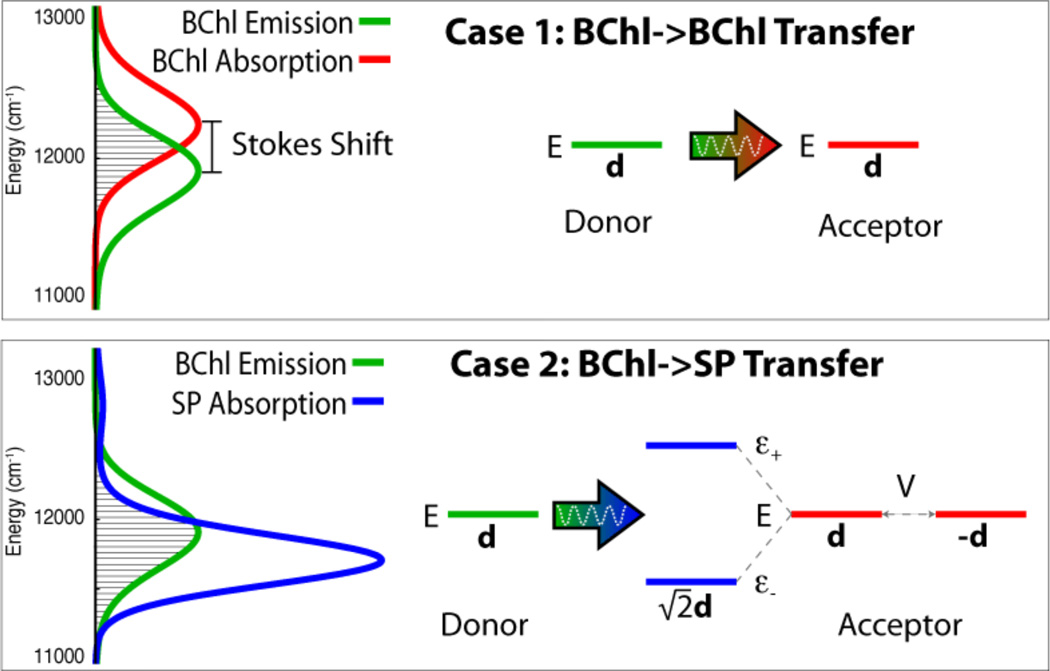
When excitation is transferred from a donor bacteriochlorophyll (BChl) to an acceptor BChl, the Stokes shift between the emission and absorption spectra causes an imperfect energy overlap, as shown in Case 1 (see filled area illustrating overlap JDA). This results in a reduced rate of excitation transfer. The reaction center can counter the reduced overlap by introducing a second acceptor BChl that is strongly coupled to the first, forming the special pair (SP). Strong coupling, accounted for by an interaction energy of V = 500 cm−1 (V is determined in Ref. 50), coherently spreads excitation between the two acceptor SP BChls, shifting also the SP exciton energies from the single BChl excited state energy E to energies ε− and ε+. This shift alters the absorption spectrum, as shown in Case 2, and accordingly increases the overlap, JDA, between emission (green line) and absorption (blue line) spectra (see filled area). Furthermore, due to the anti-parallel orientation of the SP BChl’s transition dipole moments, the lower energy exciton state attains a transition dipole moment of . The combination of better spectral overlap JDA and a stronger dA value increases the rate of excitation transfer for Case 2 over that of Case 1.