Figure 2.
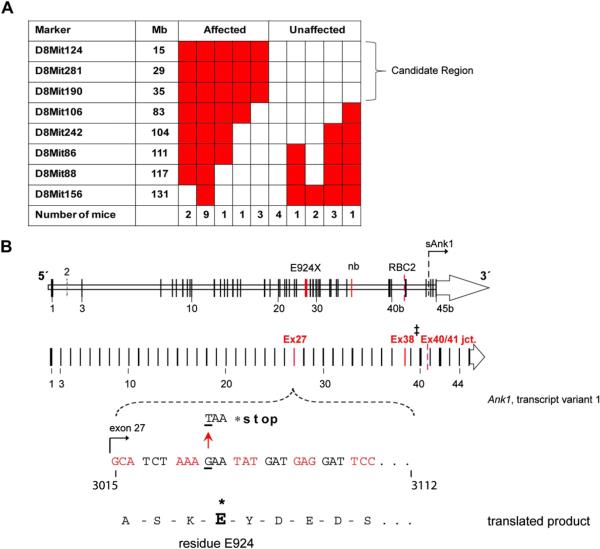
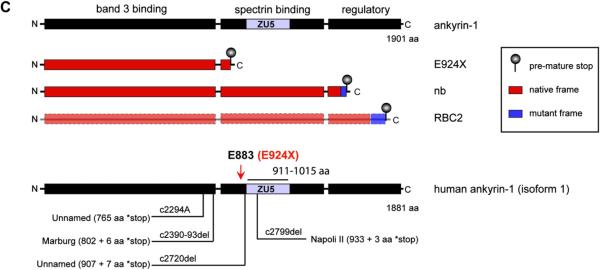
A single ENU-generated point mutation (G → T transversion) in Ank1 exon 27 yields a new mouse model of hereditary spherocytosis. (A) Table indicating the number of affected and unaffected ENU7192 mice heterozygous (red) or null (white) for Chr. 8 marker loci derived from the mutagenized strain (B6). The indicated candidate region of Chr. 8 indicated is linked to the mutant phenotype of affected mice. (B) Schematic mouse Ank1 locus highlight transcript variant 1 and the mapped position of the ENU7192 point mutation (in Ank1). A portion of Ank1 exon 27 is expanded to show the genomic sequence with the position of the ENU-generated point mutation (G → T transversion) encoding a stop codon (*) in place of glutamate (E) at residue 924 (ankyrin-1, isoform 1 [NP001104253.1]) of the theoretical translated product. The locations of the E924X point mutation within the Ank1 genomic locus relative to other reported Ank1 mutant mouse strains (Ank1nb (nb) [17] and Ank1RBC2 (RBC2) [31]) is shown. ‡Note that the nb mutation is in exon 38 using the exon numbering for Ank1, transcript variant 1(NM001110783.1) but is reported as exon 36 in the original mapping publication [17]). sAnk1 indicates the position of the alternative start site for the short ankyrin-1 isoform using the exon numbering scheme of Ank1, transcript variant 1 [12]. (C) The theoretical truncated ankyrin-1 protein products generated by the known Ank1 mouse mutant alleles. Note that ankyrin-1 protein attributable to the RBC2 allele has not been detected in reticulocytes or erythroid progenitors [31].