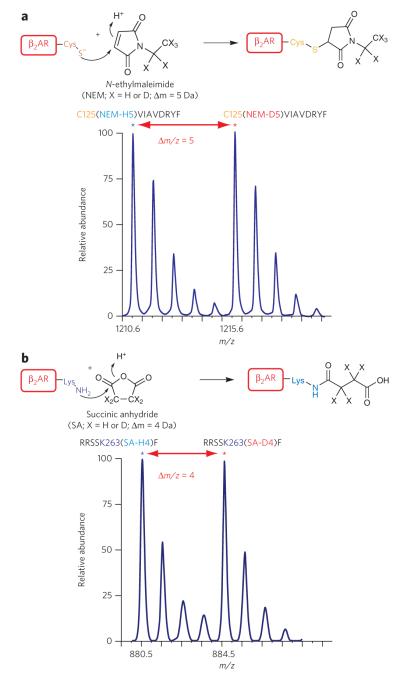
Figure 1. Labeling of cysteines and lysines in the β2AR to monitor conformational changes.
(a) top, reaction of thiolate anion of cysteine side chain with N-ethylmaleimide (neM-H5 or neM-d5) by nucleophilic addition at the double bond of the maleimide ring. bottom, representative isotope-peak pair (doublet) corresponding to a chymotryptic peptide ( 125cviAvdRYF133) modified at cys125 by a light and heavy neM (m/z 1210.6 and 1215.6, respectively; ~Δm/z = 5). (b) top, reaction of the ε-nH2 group of the lysine side chain with succinic anhydride (SA-H4 or SA-d4) by nucleophilic addition at one of the carbonyl groups. bottom, representative spectrum of doublet peaks corresponding to a chymotryptic peptide ( 259RRSSKF264) modified at lys263 by light and heavy succinic anhydride (m/z 880.5 and 884.5, respectively; ~Δm/z = 4).