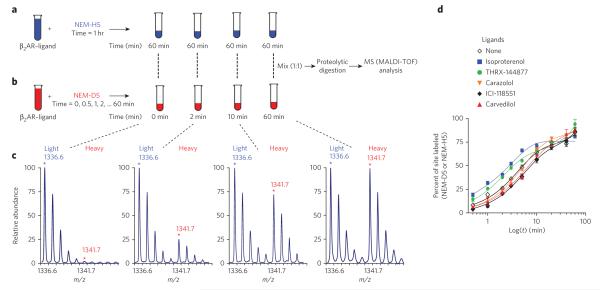
Figure 3. Schematic illustration of time-dependent, residue-specific labeling experiment designed to monitor conformational changes in the β2AR.
(a,b) Strategy for labeling of cysteines in purified β2AR, initiated in two pools by adding either neM-H5 as in (a) or neM-d5 as in (b). equal amounts of the two pools are mixed, subjected to proteolysis, and MS-analyzed to determine peptide fragments that have been modified. (c) Representative doublets with singly charged ion ([M+H]+) peaks at m/z 1336.6 and 1341.7 that correspond to peptide 327cRSpdFRiAF336 modified at cys327 by neM and exhibit a mass difference of 5 da following modification with either neM-H5 or neM-d5 (details are listed in Supplementary Methods and Supplementary Fig. 4). (d) Representative time-course curves of the extent of neM reactivity at cys327, expressed as percent of sites labeled (%F) plotted versus labeling time in minutes on a logarithmic scale, after treatment with carrier solvent or indicated ligands. the solid lines in each plot are the best fit obtained after fitting to double exponential function. data represent the average of at least three independent experiments ± s.e.m.