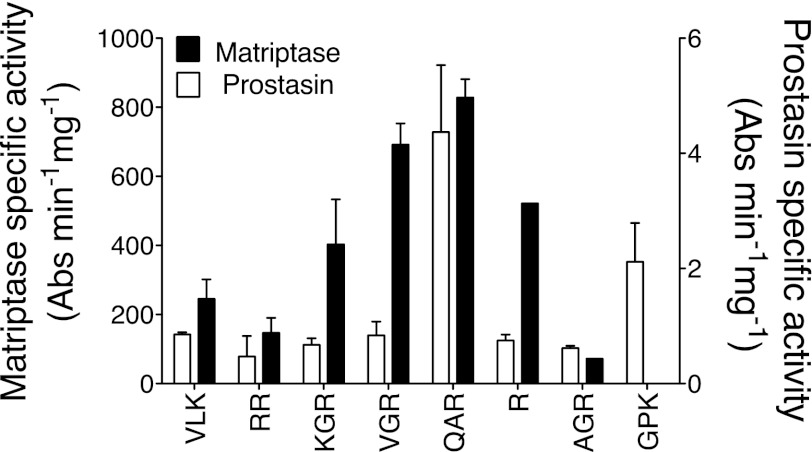
Fig. 1.
Comparison of peptidic substrate preferences of human matriptase and prostasin. Amidolytic activity was tested against soluble, recombinant proteases using 8 tryptic 4-nitroanilide (4NA) peptidic substrates, including d-Val-l-Leu-Lys-4NA (VLK), carbobenzoxy-l-Arg-Arg-4NA (RR), N-benzoyl-l-Lys-Gly-Arg-4NA (KGR), N-benzoyl-l-Val-Gly-Arg-4NA (VGR), t-butyloxycarbonyl-l-Gln-Ala-Arg-4NA (QAR), N-benzoyl-l-Arg-4NA (R), β-Ala-Gly-l-Arg-4NA (AGR), and N-(p-tosyl)-Gly-l-Pro-Arg-4NA (GPK). For each enzyme, data obtained with each substrate were normalized to specific activity [Absorbance (Abs)·min−1·mg−1 of protease] toward QAR-4NA, which was the best substrate identified for both matriptase and prostasin (N = 3).