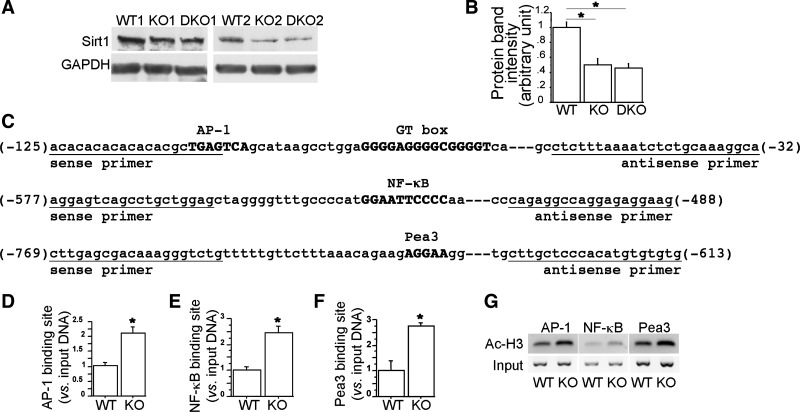
Fig. 2.
Decreased sirtuin 1 (SIRT1) expression enhanced histone H3 acetylation at the activator protein-1 (AP-1), NF-κB, and Pea3 binding sites of the MMP9 promoter in Smad3-null lungs. SIRT1 protein levels in wild-type (WT), Smad3 single knockout (KO), and Smad3/MMP9 double knockout (DKO) lungs at P28 (n ≥ 3, each) were measured by Western blots (A) and quantified by densitometry (B). GAPDH was a loading control. WT and KO lungs at P28 (n = 3, each) were collected for chromatin immunoprecipitation (ChIP) assay using anti-acetylated histone H3 antibody. The relative amount of the coimmunoprecipitated DNA at the AP-1, NF-κB, and Pea3 binding sites of the MMP9 promoter (C, sequences shown in bold uppercase), which was bound to acetylated histone H3, were quantified by real-time PCR (D–F) and also illustrated on 2% agarose gel (G). *P < 0.01.