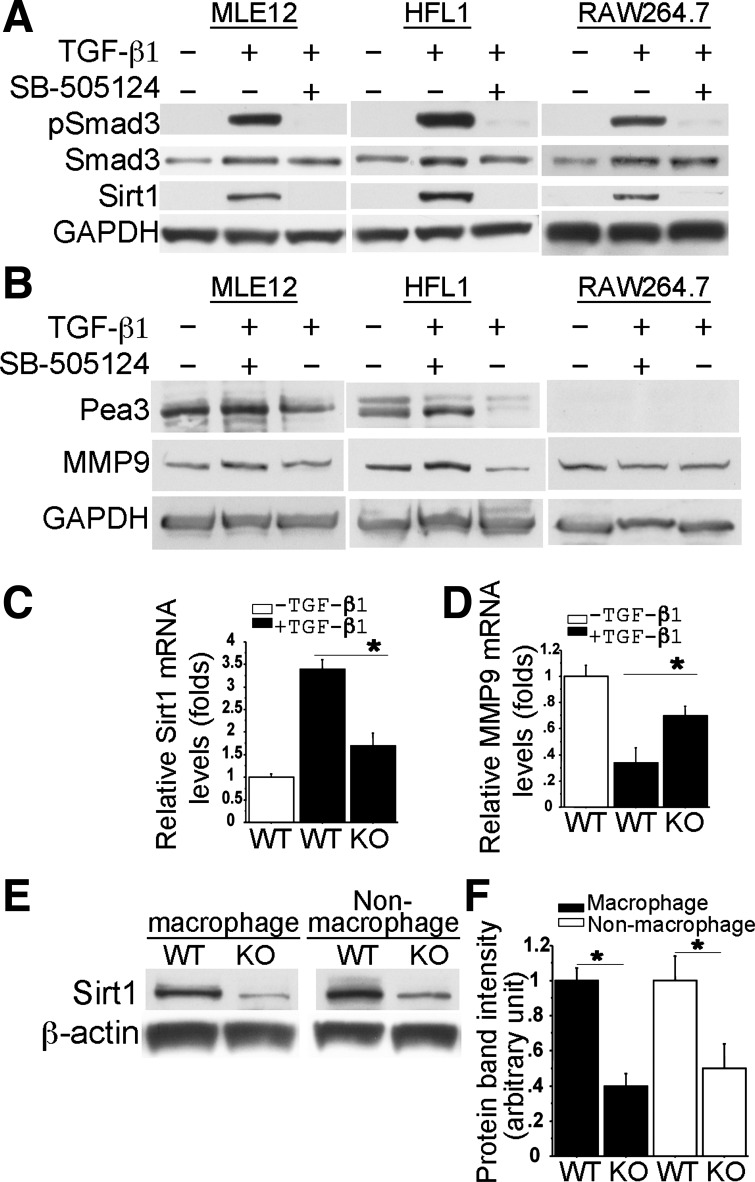
Fig. 3.
Expression of SIRT1, Pea3, and MMP9 was regulated by TGF-β1 in a variety of cells in vitro and in vivo. Western blots showed that Smad3 phosphorylation was induced 1 h after TGF-β (5 ng/ml) stimulation in mouse lung epithelial cell line 12 (MLE12), human lung fibroblast cell line 1 (HFL1), and RAW264.7 cells (A). 48 h later, induction of Pea3, SIRT1, and MMP9 in these cells was further examined by Western blot (A and B). Both pSmad3 and SIRT1 induction were abolished by pretreatment with SB-505124 (1 μM). GAPDH was a loading control (A and B). The specific induction of Sirt1 and MMP9 expression by TGF-β-Smad3 in primary macrophages, which were isolated from either WT or Smad3 KO mouse lungs, was also examined by real-time PCR after 18h TGF-β (5 ng/ml) treatment (C and D, n = 3). Alteration of endogenous SIRT1 protein levels in F4/80 antibody FACS-sorted macrophages and nonmacrophages from WT and Smad3 KO lungs were also examined by Western blot (E) and quantified by densitometry (F, n = 3) *P < 0.01.