Abstract
The complete nucleotide sequence of the coding region of the chicken carbonic anhydrase II (CA II) gene has been determined from clones isolated from a chicken genomic library. The sequence of a nearly full length chicken CA II cDNA clone has also been obtained. The gene is approximately 17 kilobase pairs (kb) in size and codes for a protein that is comprised of 259 amino acid residues. The 5' flanking region contains consensus sequences commonly associated with eucaryotic genes transcribed by RNA polymerase II. Six introns ranging in size from 0.3 to 10.2 kb interrupt the gene. The number of introns as well as five of the six intron locations are conserved between the chicken and mouse CA II genes. The site of the fourth intron is shifted by 14 base pairs further 3' in the chicken and thus falls between codons 147 and 148 rather than within codon 143 as in the mouse gene. Measurements of CA II RNA levels in various cell types suggest that CA II RNA increases in parallel with globin RNA during erythropoiesis and exists only at low levels, if at all, in non-erythroid cells.
Full text
PDF


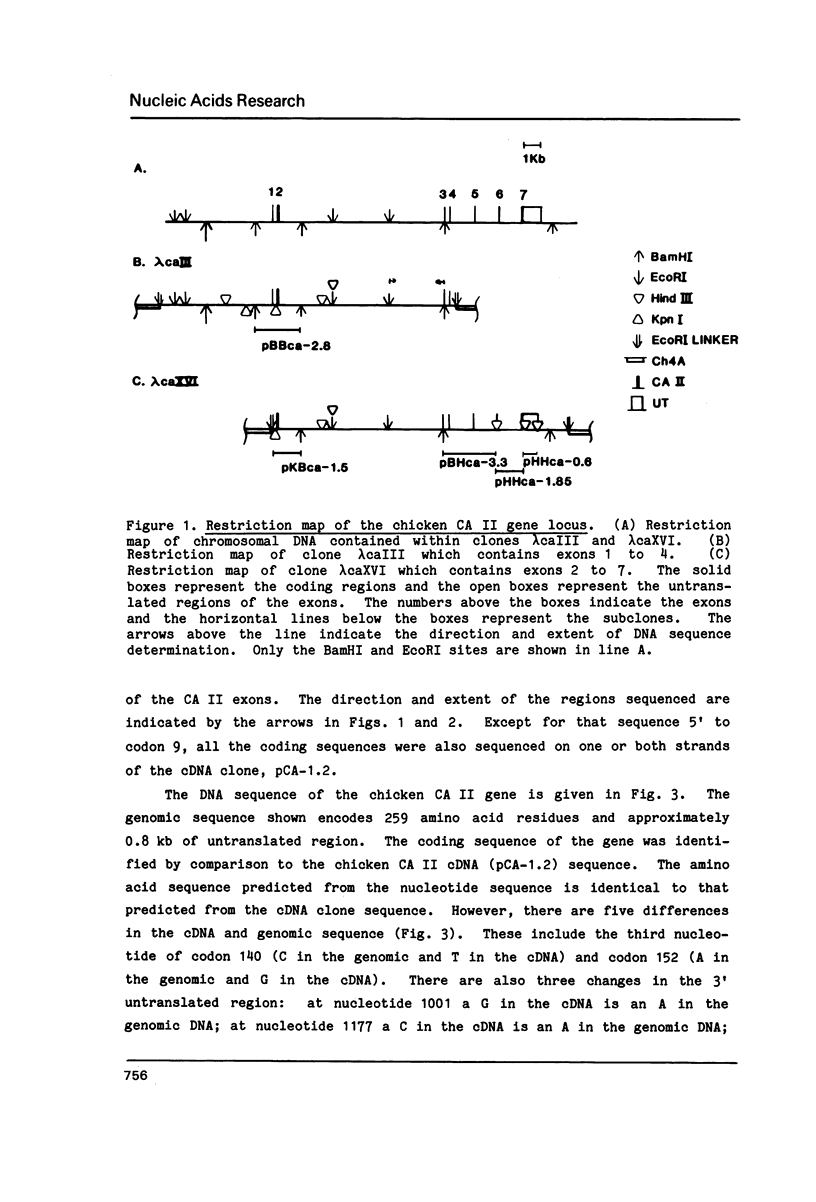
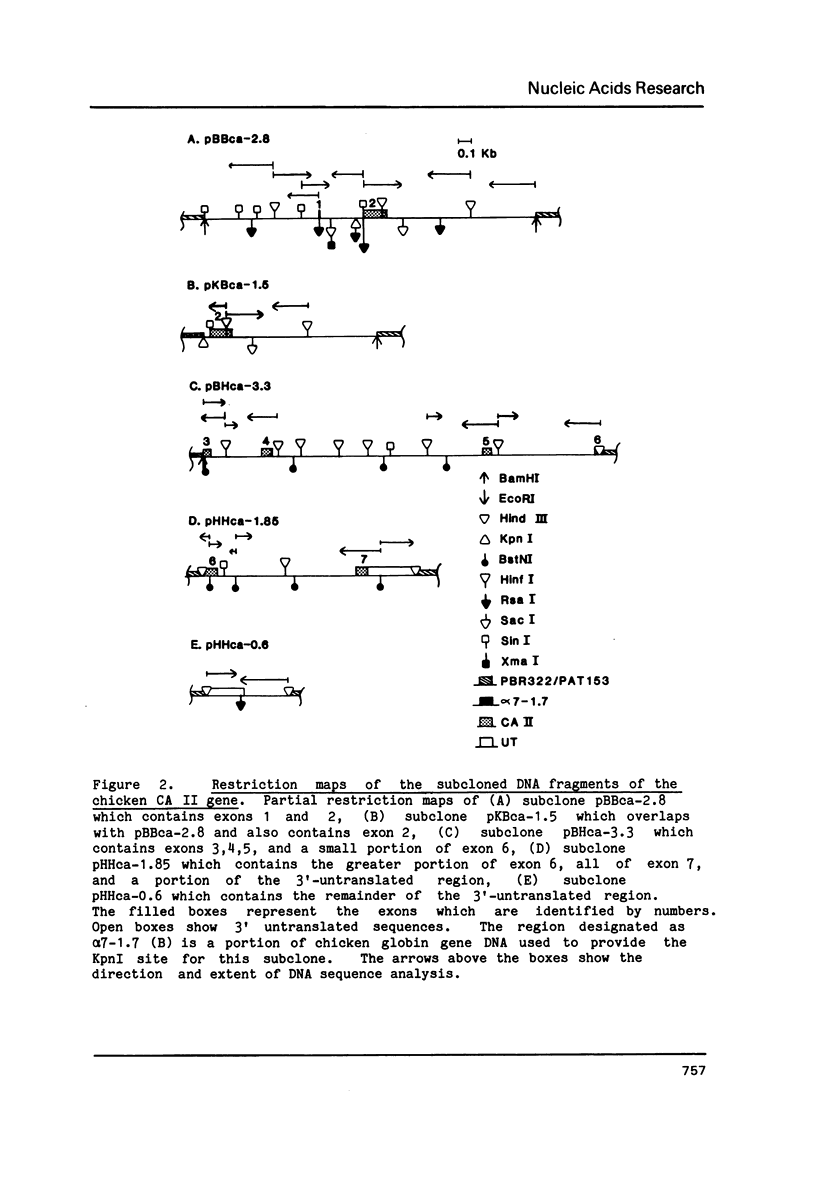



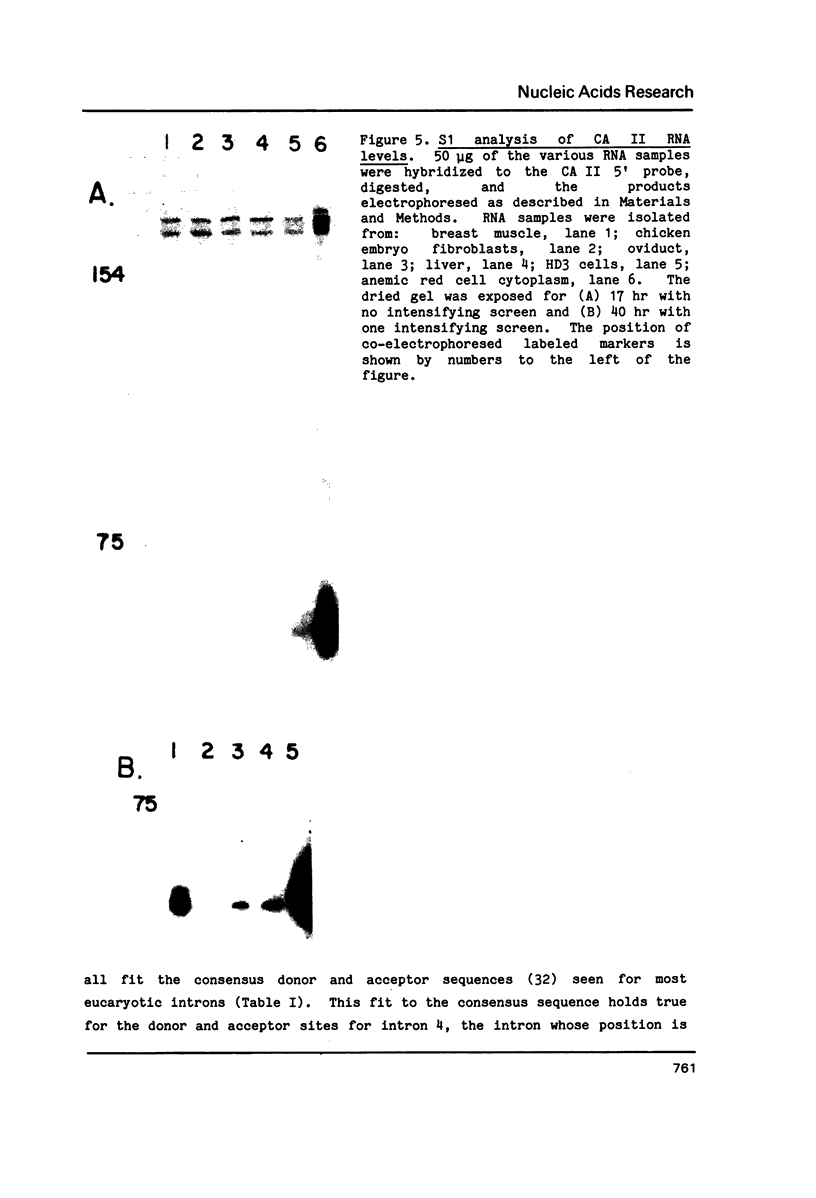









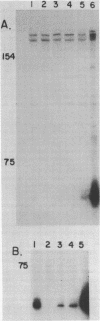
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beug H., Palmieri S., Freudenstein C., Zentgraf H., Graf T. Hormone-dependent terminal differentiation in vitro of chicken erythroleukemia cells transformed by ts mutants of avian erythroblastosis virus. Cell. 1982 Apr;28(4):907–919. doi: 10.1016/0092-8674(82)90070-8. [DOI] [PubMed] [Google Scholar]
- Boyer S. H., Ostrer H., Smith K. D., Young K. E., Noyes A. N. Isolation of cDNA clones for rabbit red cell carbonic anhydrase and catalase: a pilot study directed at isolation of coordinately expressed genes. Ann N Y Acad Sci. 1984;429:324–331. doi: 10.1111/j.1749-6632.1984.tb12356.x. [DOI] [PubMed] [Google Scholar]
- Cammer W., Fredman T., Rose A. L., Norton W. T. Brain carbonic anhydrase: activity in isolated myelin and the effect of hexachlorophene. J Neurochem. 1976 Jul;27(1):165–171. doi: 10.1111/j.1471-4159.1976.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Curtis P. J., Withers E., Demuth D., Watt R., Venta P. J., Tashian R. E. The nucleotide sequence and derived amino acid sequence of cDNA coding for mouse carbonic anhydrase II. Gene. 1983 Nov;25(2-3):325–332. doi: 10.1016/0378-1119(83)90237-8. [DOI] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Cochran M. D., Dobkin C., Reiser J., Weissmann C. Three regions upstream from the cap site are required for efficient and accurate transcription of the rabbit beta-globin gene in mouse 3T6 cells. Cell. 1983 Mar;32(3):695–706. doi: 10.1016/0092-8674(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Stadt S. J., Choi O. R., Dolan M., Fischer H. D., Engel J. D. The nucleotide sequence of the embryonic chicken beta-type globin genes. J Biol Chem. 1983 Oct 25;258(20):12685–12692. [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Dodgson S. J., Forster R. E., 2nd, Storey B. T., Mela L. Mitochondrial carbonic anhydrase. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5562–5566. doi: 10.1073/pnas.77.9.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M., Dodgson J. B., Engel J. D. Analysis of the adult chicken beta-globin gene. Nucleotide sequence of the locus, microheterogeneity at the 5'-end of beta-globin mRNA, and aberrant nuclear RNA species. J Biol Chem. 1983 Mar 25;258(6):3983–3990. [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Engel J. D., Rusling D. J., McCune K. C., Dodgson J. B. Unusual structure of the chicken embryonic alpha-globin gene, pi'. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1392–1396. doi: 10.1073/pnas.80.5.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornwald J. A., Kuncio G., Peng I., Ordahl C. P. The complete nucleotide sequence of the chick a-actin gene and its evolutionary relationship to the actin gene family. Nucleic Acids Res. 1982 Jul 10;10(13):3861–3876. doi: 10.1093/nar/10.13.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidoni D., Dynan W. S., Tjian R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. 1984 Nov 29-Dec 5Nature. 312(5993):409–413. doi: 10.1038/312409a0. [DOI] [PubMed] [Google Scholar]
- Girvitz S. C., Bacchetti S., Rainbow A. J., Graham F. L. A rapid and efficient procedure for the purification of DNA from agarose gels. Anal Biochem. 1980 Aug;106(2):492–496. doi: 10.1016/0003-2697(80)90553-9. [DOI] [PubMed] [Google Scholar]
- Hewett-Emmett D., Hopkins P. J., Tashian R. E., Czelusniak J. Origins and molecular evolution of the carbonic anhydrase isozymes. Ann N Y Acad Sci. 1984;429:338–358. doi: 10.1111/j.1749-6632.1984.tb12359.x. [DOI] [PubMed] [Google Scholar]
- Linser P., Moscona A. A. Variable CA II compartmentalization in vertebrate retina. Ann N Y Acad Sci. 1984;429:430–446. doi: 10.1111/j.1749-6632.1984.tb12369.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKinley D. N., Whitney P. L. Particulate carbonic anhydrase in homogenates of human kidney. Biochim Biophys Acta. 1976 Oct 11;445(3):780–790. doi: 10.1016/0005-2744(76)90128-5. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Samarut J., Gazzolo L. Target cells infected by avian erythroblastosis virus differentiate and become transformed. Cell. 1982 Apr;28(4):921–929. doi: 10.1016/0092-8674(82)90071-x. [DOI] [PubMed] [Google Scholar]
- Sanyal G., Pessah N. I., Maren T. H. Kinetics and inhibition of membrane-bound carbonic anhydrase from canine renal cortex. Biochim Biophys Acta. 1981 Jan 15;657(1):128–137. doi: 10.1016/0005-2744(81)90136-4. [DOI] [PubMed] [Google Scholar]
- Sapirstein V. S., Strocchi P., Gilbert J. M. Properties and function of brain carbonic anhydrase. Ann N Y Acad Sci. 1984;429:481–493. doi: 10.1111/j.1749-6632.1984.tb12375.x. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Török I. A photoinduced cleavage of DNA useful for determining T residues. Nucleic Acids Res. 1982 Dec 20;10(24):7959–7964. doi: 10.1093/nar/10.24.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. R., Calvo J. M. Nucleotide sequence of the E coli gene coding for dihydrofolate reductase. Nucleic Acids Res. 1980 May 24;8(10):2255–2274. doi: 10.1093/nar/8.10.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Venta P. J., Montgomery J. C., Hewett-Emmett D., Tashian R. E. Comparison of the 5' regions of human and mouse carbonic anhydrase II genes and identification of possible regulatory elements. Biochim Biophys Acta. 1985 Dec 18;826(4):195–201. doi: 10.1016/0167-4781(85)90006-5. [DOI] [PubMed] [Google Scholar]
- Venta P. J., Montgomery J. C., Hewett-Emmett D., Wiebauer K., Tashian R. E. Structure and exon to protein domain relationships of the mouse carbonic anhydrase II gene. J Biol Chem. 1985 Oct 5;260(22):12130–12135. [PubMed] [Google Scholar]
- Venta P. J., Montgomery J. C., Wiebauer K., Hewett-Emmett D., Tashian R. E. Organization of the mouse and human carbonic anhydrase II genes. Ann N Y Acad Sci. 1984;429:309–323. doi: 10.1111/j.1749-6632.1984.tb12355.x. [DOI] [PubMed] [Google Scholar]
- Vincent S. H., Silverman D. N. Carbonic anhydrase activity in mitochondria from rat liver. J Biol Chem. 1982 Jun 25;257(12):6850–6855. [PubMed] [Google Scholar]
- Weil S. C., Walloch J., Frankel S. R., Hirata R. K. Expression of carbonic anhydrase and globin during erythropoiesis in vitro. Ann N Y Acad Sci. 1984;429:335–337. doi: 10.1111/j.1749-6632.1984.tb12358.x. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Beug H., Groudine M., Graf T. Temperature-sensitive changes in the structure of globin chromatin in lines of red cell precursors transformed by ts-AEV. Cell. 1982 Apr;28(4):931–940. doi: 10.1016/0092-8674(82)90072-1. [DOI] [PubMed] [Google Scholar]
- Whitney P. L., Briggle T. V. Membrane-associated carbonic anhydrase purified from bovine lung. J Biol Chem. 1982 Oct 25;257(20):12056–12059. [PubMed] [Google Scholar]
- Wistrand P. J. Properties of membrane-bound carbonic anhydrase. Ann N Y Acad Sci. 1984;429:195–206. doi: 10.1111/j.1749-6632.1984.tb12333.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Yew N. S., Federspiel M., Dodgson J. B., Hayashi N., Engel J. D. Isolation of recombinant cDNAs encoding chicken erythroid delta-aminolevulinate synthase. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3702–3706. doi: 10.1073/pnas.82.11.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara C. M., Federspiel M., Dodgson J. B. Isolation of the chicken carbonic anhydrase II gene. Ann N Y Acad Sci. 1984;429:332–334. doi: 10.1111/j.1749-6632.1984.tb12357.x. [DOI] [PubMed] [Google Scholar]