Table 1.
Chemical structures of flavonoids used in this study.
| Compound | Abbreviation | Chemical Structure | Sub-classes |
|---|---|---|---|
| Naringenin | Nar | 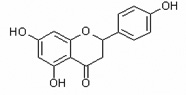 |
flavanone |
| Phloretin | Phlor | 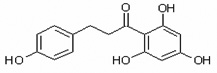 |
chalcone |
| Genistein | Gen | 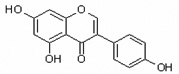 |
isoflavone |
| Apigenin 5,7,4'-Trihydroxyflavone |
Api 5,7,4'-THF |
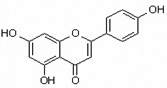 |
flavone |
| Kaempferol 3,5,7,4’tetrahydroxyflavone |
Kamp 3,5,7,4’QHF |
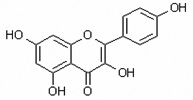 |
flavonol |
| 4'-Hydroxyflavone | 4HF | 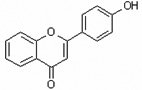 |
flavones |
| 5-Hydroxyflavone | 5HF | 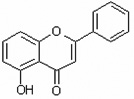 |
|
| 7-Hydroxyflavone | 7HF | 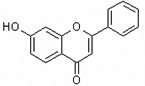 |
|
| 5,4'-Dihydroxyflavone | 5,4’DHF | 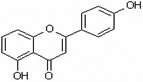 |
|
| 5,7-Dihydroxyflavone | 5,7DHF | 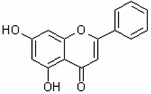 |
|
| 7,4'-Dihydroxyflavone | 7,4’DHF | 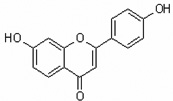 |
|
| 3-Hydroxyflavone | 3HF | 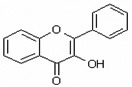 |
flavonols |
| 3,4'-Dihydroxyflavone | 3,4’DHF | 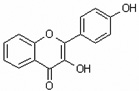 |
|
| 3,7-Dihydroxyflavone | 3,7DHF | 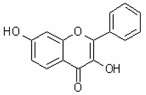 |
|
| 3,5,7-Trihydroxyflavone | 3,5,7THF | 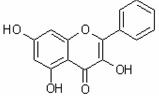 |
|
| 3,7,4'-Trihydroxyflavone | 3,7,4’THF | 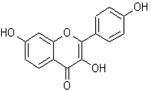 |
