Summary
There are three major treatment options for cerebral AVMs; surgery, embolization and radiosurgery. Embolization may be effective to reduce the size and density but completely obliterates AVMs only in a minority of cases. Radiosurgery may be an alternative to resection, especially in smaller AVMs. Large AVMs have been considered difficult to treat safely and effectively with single fraction radiosurgery. Hypofractionated conformal stereotactic radiotherapy (HCSRT) alone or in combination with embolization may be an alternative treatment. Embolization may reduce the volume and density of AVMs, followed by HCSRT, allowing a safe delivery of a higher total dose of radiation than possible with a single fraction. Sixteen patients with AVMs were treated with embolization and HCSRT. Embolization was performed in 1-6 (median 2) sessions. HCSRT was delivered in 5 fractions with 6-7 Gy each to the total dose of 30-35 Gy. Cerebral angiographies before and after embolization were digitally compared for calculation of volume reduction and luminescence as a measure of AVM density. The mean AVM volume in 15 patients was reduced from 11.9 ± 2.1 (1-29, median 10.0) ml to 6.5 ± 2.0 (0.5-28, median 3) ml by embolization. The luminescence for all AVMs was significantly higher after than before embolization, indicating that all AVMs were less dense after embolization. Thirteen out of 16 patients (13/16, 81%) treated with embolization and HCSRT have so far shown obliteration of their AVMs 2-9 (median 4) years after HCSRT. Three patients experienced neurological sequele after embolization, and three patients developed radionecrosis after HCSRT. Using a new method to compare cerebral angiographies in AVMs we report reduction in density and volume after embolization. The obliteration rate of a combined treatment with embolization and HCSRT seems comparable with single fraction radiosurgery although the AVMs in our series are larger than reported in most series treated with single fraction radio surgery.
Key words: AVM, embolization, stereotactic, radiotherapy
Introduction
The successful treatment of large AVMs still remains a challenge. Surgery is an option but is associated with a high risk of mortality and morbidity in large AVMs, especially in eloquent areas1. Radiosurgery and embolization are two other treatment options. Various combinations of the three treatment options have been reported2-5. Embolization may be effective to reduce the size and density of the AVM, but reaches the goal of total obliteration only in a minority of cases. The rate of complete obliteration after embolization has been reported to range from 5-22% by most authors6, although a success rate of complete obliteration has been reported to be as high as 40%7. Radiosurgery may be an alternative to resection, especially in AVMs smaller than 2 cm8. Large AVMs, however, have been considered difficult to treat safely and effectively radiosurgery. The two year obliteration rate of AVMs larger than 4 ml is reported to be 4058%, in comparison to smaller AVMs where the obliteration frequency is reported to be 85-100%9,10. Radiation necrosis in patients treated with radiosurgery is reported to range from 3 to 6% 10. Treatment with hypofractionated conformal stereotactic radiotherapy (HCSRT) may be an alternative in large AVMs, allowing a safe delivery of a higher total dose of radiation than what is possible with a single fraction11. In this article we report the results concerning reduction in size, density and obliteration in a series of patients treated with a combination of embolization and HCSRT.
Material and Methods
Patients
Between 1988 and 2001 sixteen patients (9 female, 7 male) with cerebral AVMs were treated with a combination of embolization and HCSRT. Two of these patients were treated surgically with devascularisation of the AVM prior to embolization. The embolizations were performed at Sahlgrenska University Hospital. Fifteen patients received HCSRT after the last session of embolization and in one patient embolization was performed after treatment with HCSRT. Mean age during treatment with HCSRT was 36.2 (16-62) years. The clinical presentation was intracerebral haemorrhage in five patients, subarachnoidal haemorrhage in two patients and epileptic seizures in five patients. Four other patients had headaches and vertigo as initial manifestations of their AVMs. Five AVMs were located in the parietal lobe, four in the frontal lobe, three in the occipital lobe, two in the temporal lobe, one in the cerebellar hemisphere and one centrally located in the brain stem.
The Spetzler and Martin grading system was used to classify all AVMs according to size, location in eloquent areas and venous drainage 12, (table 1).
Table 1.
AVMs graded according to the Spetzler-Martin classification.
| Spetzler-Martin Grade / No of patients | |||||||
| I | 4 | II | 4 | III | 5 | IV | 3 |
Embolization
The embolization was performed as described previously with N-butyl cyanoacrylate 13. The endovascular procedures were staged with 4 to 6 weeks between sessions. The 15 patients that were embolized prior to HCSRT were treated in 1-6 (median 2) sessions. The aim was to close the AVM totally or to decrease the total volume as much as could be safely achieved. For measurements of volume the AVM was outlined on angiographic films before and after the series of embolization procedures. Only the nidus area, without feeding arteries and draining veins were included. Three perpendicular diameters were measured and multiplied, the product divided by 2, giving the volume in ml14.
Hypofractionated Conformal Stereotactic Radiotherapy
The procedure for HCSRT has earlier been described 11. To allow hypofractionation the relocatable Laitinen stereoadapter was used 15,16. Stereotactic irradiation was delivered in five fractions with 4-11 conformal fields with individually cast shielding blocks. Irradiation was performed with 6 MV photons from a Linear accelerator (Dynaray 6H, BBC or later Varian Clinac 2300CD). The size and arrangement of the irradiation fields were adapted so that the target volume was encompassed by the 90% isodose when normalized to the isocenter. Patients were treated every second day or in five consecutive days with a dose of 6-7 Gy in each fraction to a total dose ranging from 30-35 (median 32.5) Gy. In one case an additional treatment was given with 10 Gy in a single dose because of only partial obliteration at the angiographic follow-up two years after the treatment.
Image Analysis
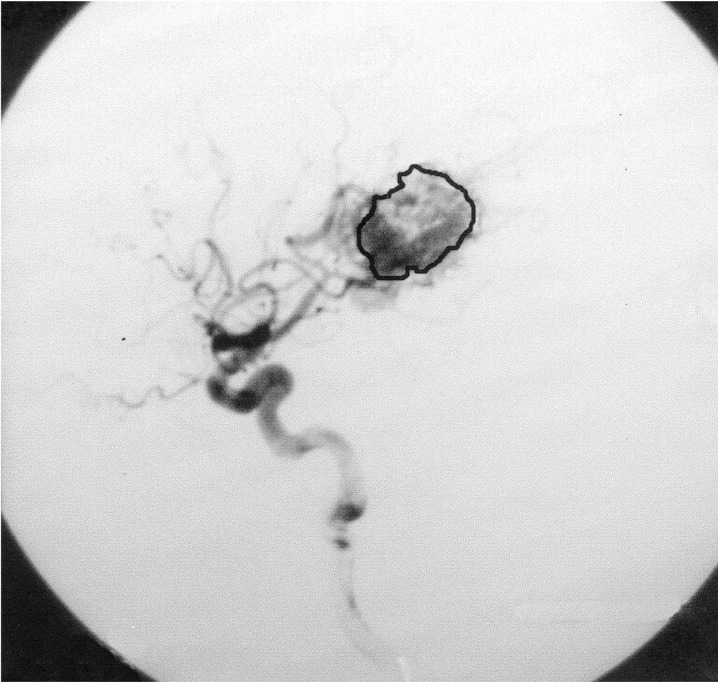
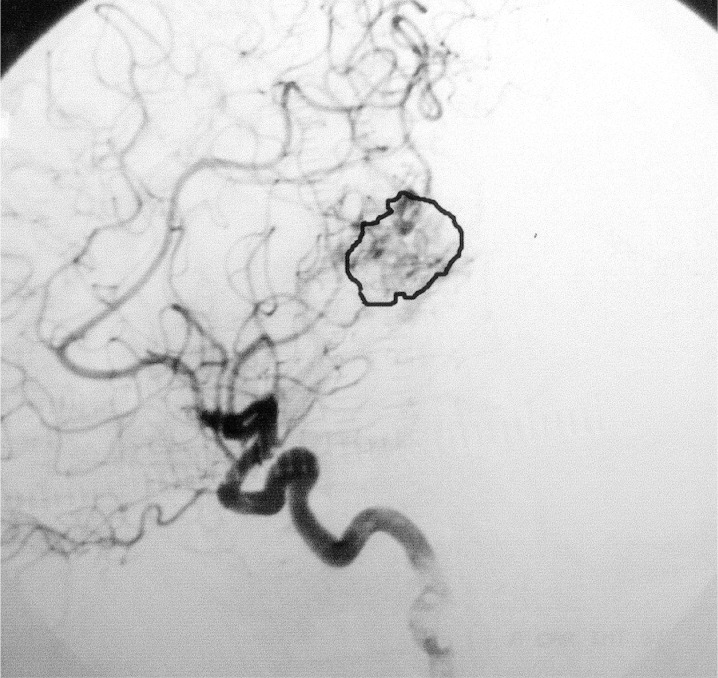
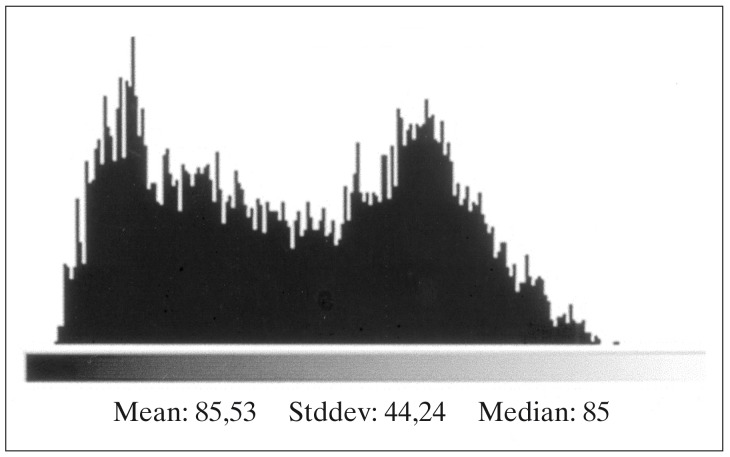
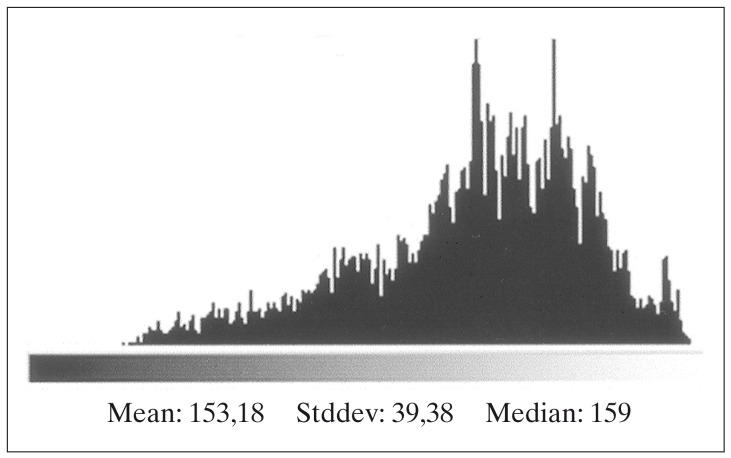
X-Ray films from cerebral angiographies performed before and after embolization were photographed using a digital camera (Ixus 330, Canon®). The post embolization angiography was performed prior to HCSRT, typically six months after embolization. Digital images from each patient before and after treatment with embolization were compared using the software Adobe Photoshop 7.0 (Adobe Systems Inc, CA, USA). Digital images were transformed into the same level of grey-scale and size and superposed over each other. Images from before and after embolization were chosen when there was maximal filling of contrast in the nidus of the AVM. The lateral projections of a carotid angiography and in three cases the lateral projections of a vertebral angiography were used for comparison before and after embolization. Correct superposition of the AVMs was achieved by superposition of vessels visible both before and after embolization. The AVMs were delineated on images performed before and after embolization using the pre embolization image as a template (figures 1,2). Mean luminescence for the delineated area on the images pre and post embolization was attained (figures 3,4). An Index for luminescence was created for comparison of the two images (luminescence after embolization/luminescence before embolization). An Index >1 would mean that the area delineating the AVM was brighter, indicating a less dense vascularity after the embolization than before.
Figure 1.
Angiography of the left ICA, lateral view, before embolization.
Figure 2.
Angiography of the left ICA, lateral view, after embolization.
Figure 3.
Luminescence of the delineated area in figure 1.
Figure 4.
Luminescence of the delineated area in figure 2.
Statistical Analysis
The difference in volume and mean luminescence before and after embolization was evaluated and tested for significance using the Wilcoxon signed rank test.
Results
Embolization Reduction in Size
Fifteen patients were treated with embolization prior to HCSRT. The mean AVM volume in these patients before embolization was 11.9 ± 2.1 (1-29, median 10.0) ml. The mean volume after endovascular treatment was 6.5 ± 2.0 (0.5-28, median 3) ml. This difference showed to be significant, p<0.005. If two large AVMs (29 and 20 ml) where the volume of the nidus was not really changed (but a significant reduction of vascular density was obtained) are omitted the mean volume reduction was from 10.0 ± 1.9 (1-25, median 10.0) ml to 3.6 ±0.7 (0.5-8, median 3.0) ml.
Embolization-Reduction in Density
The luminescence for all AVMs was higher after than before treatment with embolization, indicating that all AVMs were less dense after this treatment (table 2). The mean Index of luminescence was 1.77 (1.01-3.25). The difference in luminescence before and after embolization showed to be significant, p<0.005.
Table 2.
AVM volume and luminescence in 15 patients treated with embolization prior to HCSRT.
| Pat No | AVM volume a (ml) |
AVM volume b (ml) |
Mean Luminescencea |
Mean Luminescence b |
Luminescence Index |
Outcome |
| 1 | 4 | 3 | 126.20 | 156.25 | 1.24 | CO |
| 2 | 8 | 2 | 180.84 | 182.1 | 1.01 | CO |
| 3 | 15 | 5 | 83.82 | 171.16 | 2.04 | CO |
| 4 | 3 | 0.5 | 56.43 | 97.41 | 1.73 | CO |
| 5 | 10 | 5 | 52.30 | 170.05 | 3.25 | PO |
| 6 | 6 | 2 | 90.57 | 120.55 | 1.33 | CO |
| 7 | 10 | 3 | 85.53 | 153.18 | 1.78 | CO |
| 8 | 6 | 2 | 118.33 | 154.99 | 1.31 | CO |
| 9 | 12 | 3 | 123.83 | 158.60 | 1.28 | CO |
| 10 | 10 | 8 | 79.16 | 122.33 | 1.55 | CO |
| 11 | 25 | 6 | 40.28 | 94.98 | 2.36 | CO |
| 12 | 20 | 22 | 33.03 | 89.54 | 2.71 | CO |
| 13 | 20 | 7 | 49.04 | 113.1 | 2.31 | CO |
| 14 | 1 | 1 | 79.02 | 141.51 | 1.79 | PO |
| 15 | 29 | 28 | 116.10 | 147.53 | 1.27 | PO |
| Mean | 11.9 | 6.5*** | 88.61 | 138.27*** | 1.77 | |
|
p <0.005=*** a Before embolization - b After embolization. - c CO = Completely obliterated, PO = Partially obliterated and still followed. | ||||||
Hypofractionated Conformal Stereotactic Radiotherapy
The mean AVM volume in 15 patients after endovascular embolization and prior to treatment with HCSRT treatment was 6.5 ± 2.0 (0.528, median 3) ml. One patient with an AVM in combination with an arteriovenous dural fistula was treated with HCSRT and subsequently with surgical devascularisation and peroperative embolization.
The residual volume of this AVM was estimated to be 4.5 ml after this treatment. Thirteen out of 16 patients (13/16, 81%) treated with a combination of embolization and HCSRT have so far shown complete obliteration of their AVMs 2-9 (median 4) yrs after HCSRT.
Three patients with only partial obliteration of their AVMs are still being followed up with cerebral angiographies at two year intervals. Two of these patients are awaiting angiographic follow-up four years after treatment with HCSRT, and one patient who was retreated with 10 Gy in a single dose 3.5 years after the first treatment is awaiting angiographic follow up two years after the retreatment.
Complications
At embolization three patients experienced complications that gave clinical symptoms. Two of them had low level pareses (one monoparesis of a hand and one hemiparesis). The third patient had a hemisensory syndrome. In these three patients symptoms improved over time. Minor symptoms that did not significantly altered the patients lifestyle remained in all three patients.
After HCSRT three patients out of 16 (3/16, 19%), with a mean AVM volume of 20.3 ml experienced symptomatic radionecrosis. MRI showed signs of necrosis and edema. Two patients with parietal AVMs (12 and 29 ml) developed a transient spastic hemiparesis on the contralateral side, the third patient with a frontal AVM (20 ml) developed signs of a frontal lobe syndrome. All three patients responded to treatment with corticosteroids.
Discussion
In this study we report reduction in size as well as density in AVMs treated with embolization prior to treatment with HCSRT. Most authors that report results from embolization of AVMs have focused mainly on the volume reduction of the AVMs 5,17-19, maybe due to the difficult task of assessing the reduction in density. From our point of view it is clear that a substantial effect of embolization is the reduction in density apart from the volume reduction. We have tried to assess this reduction in density in a more objective way using modern software for image analysis and to superpose AVMs before and after embolization. Luminescence has been used as a measure of the density of the AVM. There are however factors that may influence the measurements of luminescence and bias the analysis.
Angiographies are dynamic investigations, and it is difficult to attain angiographic X-ray film for comparison in the exactly same arteriovenous phase pre and post embolization. The embolization may also change the flow through the nidus
In this study we have chosen to compare images pre and post embolization with maximal filling of contrast in the nidus of the AVM. Contrast medium with different concentration of Iodine may also have been used in the angiographies performed before and after embolization, resulting in a false difference in luminescence. It is also possible that embolization material visible on the angiography performed after the embolization may obscure contrast enhancement from a residual AVM, resulting in a falsely higher luminescence.
Concerning AVM volume there was a discrepancy between the estimated residual AVM volumes after embolization calculated from measurements done on X-ray film according to the method described14 and the volumes of the AVMs determined by our three-dimensional dose planning system (TMS. MDS Nordion) prior to HCSRT. In most cases the estimated volume was higher using the 3-D dose planning system. We have chosen to report volumes estimated as described above to be sure not to exaggerate AVM volumes despite the fact that volume estimation using a 3-D dose planning system may be a more accurate method. When determining AVM volume with a 3-D doseplanning system, from coordinates derived from a stereotactic angiography and a stereotactic CT scan with the Laitinen adapter applied, the volume can be correctly estimated even from an irregular shaped AVM. In the method reported above the volume is calculated using perpendicular diameters and assuming a ellipsoid shape of the AVM. The uncertainty of magnification factors in X-ray film may also contribute to an error in the volume estimation.
Eighty-one percent of the AVMs treated with a combination of embolization and HCSRT have so far showed obliteration 2-9 years after treatment. Comparing with single fraction radiosurgery, one study has reported a two year obliteration rate of 85% in AVMs 1-4 ml and 58% in AVMs 4-10 ml9. The result of AVM treatment is among other factors related to the size of the AVM, and most authors have reported that the obliteration rate is inversely correlated with the volume of the AVM or the maximum diameter of the nidus 20. Considering radiobiology there is a potential for therapeutic gain with hypofractionated radiotherapy, allowing a higher dose of irradiation than possible with a single fraction 21. This is clinically important when AVMs are large and/or located in eloquent areas. Thus, a combination of embolization and HCSRT may be an alternative in the treatment of large AVMs.
Delayed radiation-induced complications have been reported by all authors performing radiosurgery to treat AVMs. Symptomatic radiation necrosis after radiosurgery has been reported to occur in 3-6% 10.
The rate of symptomatic radionecrosis in this study is substantially higher (3/16, 19%) and occurred in patients with large AVM (12, 20, 29 ml, mean 20.3 ml. This may indicate that our present treatment model with 30-35 Gy given in five fractions is in the upper dose range for treating volumes of 0.5-20 ml and especially then the larger AVMs in our series. However, the patients reported in this study constitute a subgroup of patients treated with HCSRT. The entire group of patients have, as reported elsewhere 11, experienced symptomatic radionecrosis in 3.4% of the cases, which is in the range of rates reported from other series.
Conclusions
We report the results of a combined treatment of AVMs with embolization and HCSRT in terms of volume reduction, reduction in density and obliteration. Most authors have reported volume reduction of AVMs following embolization. We have focused on the reduction of density as well as volume after embolization and we describe a new objective method to evaluate reduction in vascular density using luminescence as a measure. The results of combined treatment with embolization and HCSRT in terms of obliteration seem comparable with single fraction radiosurgery although our AVMs were larger than in most series reported with single fraction radiosurgery. We believe that in large AVMs a combined treatment with embolization and HCSRT may be an attractive option resulting in a high frequency of obliteration.
References
- 1.Soderman M, Andersson T, et al. Management of patients with brain arteriovenous malformations. Eur J Radiol. 2003;3:195–205. doi: 10.1016/s0720-048x(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 2.Sano H, Kato Y, et al. Strategy for the treatment of arteriovenous malformations. J Clin Neurosci. 2000:60–68. doi: 10.1054/jocn.2000.0714. [DOI] [PubMed] [Google Scholar]
- 3.Flickinger JC, Kondziolka D, et al. An analysis of the dose-response for arteriovenous malformation radiosurgery and other factors affecting obliteration. Radiother Oncol. 2002;3:347–354. doi: 10.1016/s0167-8140(02)00103-2. [DOI] [PubMed] [Google Scholar]
- 4.Jizong Z, Shuo W, et al. Combination of intraoperative embolization with surgical resection for treatment of giant cerebral arteriovenous malformations. J Clin Neurosci. 2000:54–59. doi: 10.1054/jocn.2000.0713. [DOI] [PubMed] [Google Scholar]
- 5.Chang SD, Marcellus ML, et al. Multimodality treatment of giant intracranial arteriovenous malformations. Neurosurgery. 2003;1:1–11. doi: 10.1227/01.neu.0000068700.68238.84. discussion 11-13. [DOI] [PubMed] [Google Scholar]
- 6.Yu SC, Chan MS, et al. Complete obliteration of intracranial arteriovenous malformation with endovascular cyanoacrylate embolization: initial success and rate of permanent cure. Am J Neuroradiol. 2004;7:1139–1143. [PMC free article] [PubMed] [Google Scholar]
- 7.Valavanis A CG. Endovascular management of cerebral arteriovenous malformations. Neurointerventionist. 1999:34–40. [Google Scholar]
- 8.Aoyama H, Shirato H, et al. Treatment outcome of single or hypofractionated single-isocentric stereotactic irradiation (STI) using a linear accelerator for intracranial arteriovenous malformation. Radiother Oncol. 2001;3:323–328. doi: 10.1016/s0167-8140(01)00303-6. [DOI] [PubMed] [Google Scholar]
- 9.Lunsford LD, Kondziolka D, et al. Stereotactic radiosurgery for arteriovenous malformations of the brain. J Neurosurg. 1991;4:512–524. doi: 10.3171/jns.1991.75.4.0512. [DOI] [PubMed] [Google Scholar]
- 10.Friedman WA, Bova FJ, et al. Linear accelerator radiosurgery for arteriovenous malformations: the relationship of size to outcome. J Neurosurg. 1995;2:180–189. doi: 10.3171/jns.1995.82.2.0180. [DOI] [PubMed] [Google Scholar]
- 11.Lindvall P, Bergstrom P, et al. Hypofractionated conformal stereotactic radiotherapy for arteriovenous malformations. Neurosurgery. 2003;5:1036–1042. doi: 10.1227/01.neu.0000088566.82699.e6. discussion 1042-1033. [DOI] [PubMed] [Google Scholar]
- 12.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;4:476–483. doi: 10.3171/jns.1986.65.4.0476. [DOI] [PubMed] [Google Scholar]
- 13.Wikholm G, Lundqvist C, et al. Embolization of cerebral arteriovenous malformations: Part I - Technique, morphology, and complications. Neurosurgery. 1996;3:448–457. doi: 10.1097/00006123-199609000-00004. discussion 457-449. [DOI] [PubMed] [Google Scholar]
- 14.Pasqualin A, Barone G, et al. The relevance of anatomic and haemodynamic factors to a classification of cerebral arteriovenous malformations. Neurosurgery. 1991;3:370–379. doi: 10.1097/00006123-199103000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Laitinen LV, Liliequist B, et al. An adapter for computed tomography-guided stereotaxis. Surg Neurol. 1985;6:559–566. doi: 10.1016/0090-3019(85)90003-5. [DOI] [PubMed] [Google Scholar]
- 16.Hariz MI, Eriksson AT. Reproducibility of repeated mountings of a noninvasive CT/MRI stereoadapter. Appl Neurophysiol. 1986;6:336–347. doi: 10.1159/000100163. [DOI] [PubMed] [Google Scholar]
- 17.Soderman M, Rodesch G, et al. Gamma knife outcome models as a reference standard in the embolization of cerebral arteriovenous malformations. Acta Neurochir (Wien) 2001;8:801–810. doi: 10.1007/s007010170034. [DOI] [PubMed] [Google Scholar]
- 18.Miyachi S, Negoro M, et al. Embolization of cerebral arteriovenous malformations to assure successful subsequent radiosurgery. J Clin Neurosci. 2000:82–85. doi: 10.1054/jocn.2000.0718. [DOI] [PubMed] [Google Scholar]
- 19.Lundqvist C, Wikholm G, et al. Embolization of cerebral arteriovenous malformations: Part II--Aspects of complications and late outcome. Neurosurgery. 1996;3:460–467. doi: 10.1097/00006123-199609000-00005. discussion 467-469. [DOI] [PubMed] [Google Scholar]
- 20.Chang JH, Chang JW, et al. Factors related to complete occlusion of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 2000:96–101. doi: 10.3171/sup.2000.93.supplement3.0096. [DOI] [PubMed] [Google Scholar]
- 21.Wigg DR. Is there a role for fractionated radiotherapy in the treatment of arteriovenous malformations? Acta Oncol. 1999;8:979–986. doi: 10.1080/028418699432220. [DOI] [PubMed] [Google Scholar]