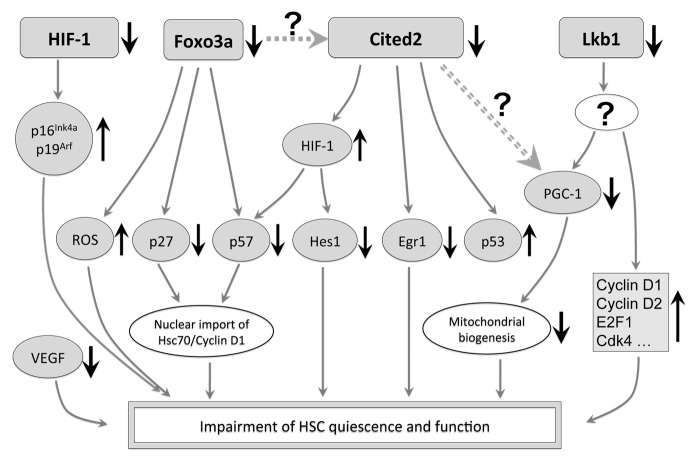
Figure 1. Molecular network of Cited2 and its associated players in the regulation of HSC quiescence. HIF-1 maintains the quiescence of HSCs partly via the repression of p16Ink4a and p19Arf. Although the deletion of HIF-1α does not affect the expression of Vegf, the lack of VEGF results in impaired HSC reconstitution capacity. The deficiency of Cited2 results in downregulation of multiple HSC quiescence related genes, including Egr1, p57kip2 and Hes1. The downregulation of p57kip2 and Hes1 is most likely mediated by the elevated activity of HIF-1, as additional deletion of HIF-1α in Cited2-knockout mice largely resumes the expression of p57kip2 and Hes1 to normal levels. Previous reports also showed increased activity of p53 in Cited2-knockout HSCs. Foxo3a regulates HSC quiescence mainly by repression of reactive oxygen species (ROS) and activation of p27kip1 and p57kip2. Although Foxo3a has been shown to induce the transcription of the Cited2 gene in fibroblasts (dashed line), whether Cited2 is downstream of Foxo3a in HSCs requires further investigation. Lkb1 regulates HSC cell cycle progression and mitochondrial biogenesis partly by the regulation of PGC-1 in an AMPK- and mTOR-independent manner, but the underlying mechanism remains unknown. Cited2 positively regulates the activity of PGC-1α in hepatic cells (double dashed line), and the significance of this regulation in HSC metabolism needs to be further pursued. Upward and downward arrows stand for increased and decreased activities, respectively.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.