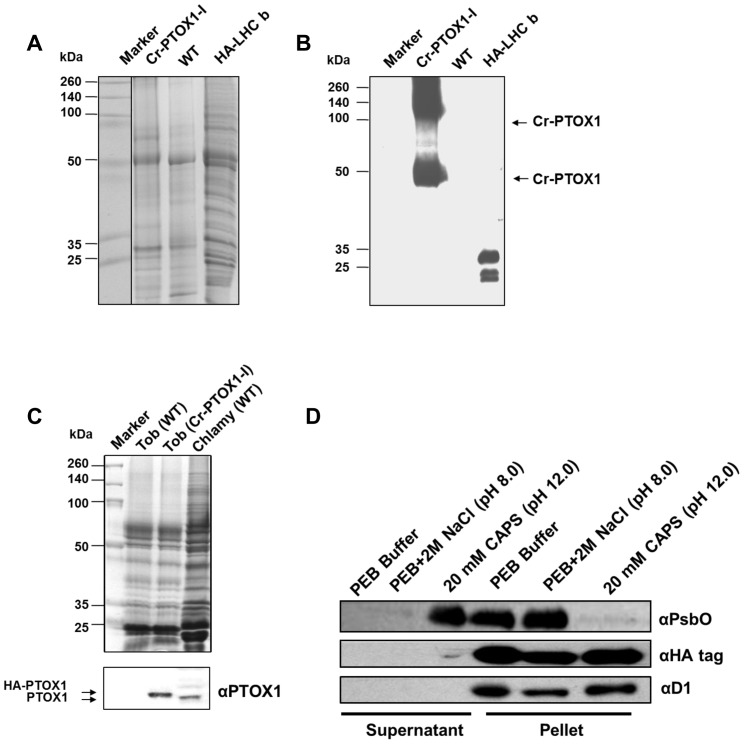
Figure 3. Detection of Cr-PTOX1 by SDS-PAGE and Western blot analysis.
Total proteins equivalent to 1 µg of chlorophyll were loaded per well from PTOX1-I plant leaves grown in a greenhouse at high light (125 µmol photons m−2 s−1) and analysed either by running on a 15% (w/v) denaturing polyacrylamide gel and stained by Coomassie Blue (A) or transferring to PVDF for immunodetection carried out using an anti-HA tag antibody (B). Protein samples from Chlamydomonas reinhardtii expressing HA-tagged Light Harvesting Complex b (HA-LHC b) [49] were used as a positive immunoblotting control. (C) Tobacco plants were grown in a growth room at 50 µmol photons m−2 s−1. 5 µg of thylakoids (based on chlorophyll) from tobacco, wild type (WT) as well Cr-PTOX1 expressing plants and C. reinhardtii (wild type) were loaded per well for SDS-PAGE and immunoblotting, which was carried out using an anti-PTOX1 antibody. Upper panel shows a Coomassie-stained gel, whereas, the bottom panel shows the immunoblot. (D) Differential extraction of thylakoids to determine whether Cr-PTOX1 is being targeted to the membrane. Thylakoids extracted from Cr-PTOX1-I plant leaves were washed with different buffers and centrifuged. The supernatant and pellet fractions (∼1 µg chlorophyll per well) were loaded and immunoblotted using different antibodies against membrane bound proteins.