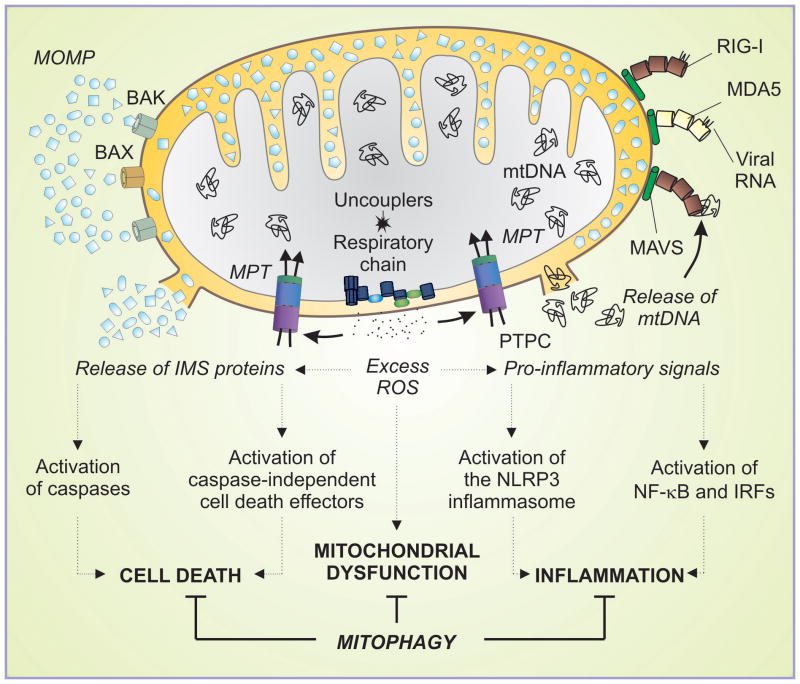
Figure 3. Mitophagy exerts cytoprotective effects by intercepting lethal signals before or at the level of mitochondria.
In response to lethal stimuli, mitochondria can undergo BAX- or BAK-mediated mitochondrial outer membrane permeabilization (MOMP), or activate the permeability transition pore complex (PTPC), driving the mitochondrial permeability transition (MPT). In both instances, intermembrane space proteins (IMS) are released into the cytosol where they activate caspase-dependent and -independent mechanisms that mediate cell death. One MPT trigger is represented by reactive oxygen species (ROS), which can be generated upon respiratory chain uncoupling. Production of mitochondrial ROS can trigger the NALP3 inflammasome via an unknown mechanism. In some settings, ROS-mediated MPT may favor the release of mitochondrial DNA (mtDNA), which can activate stimulate pro-inflammatory signaling via RIG-I and MDA5, both of which function as viral RNA sensors and interact with mitochondria through the adaptor MAVS. Activated RIG-I and MDA5 promote the activation of NF- κB and interferon regulatory factors (IRFs).