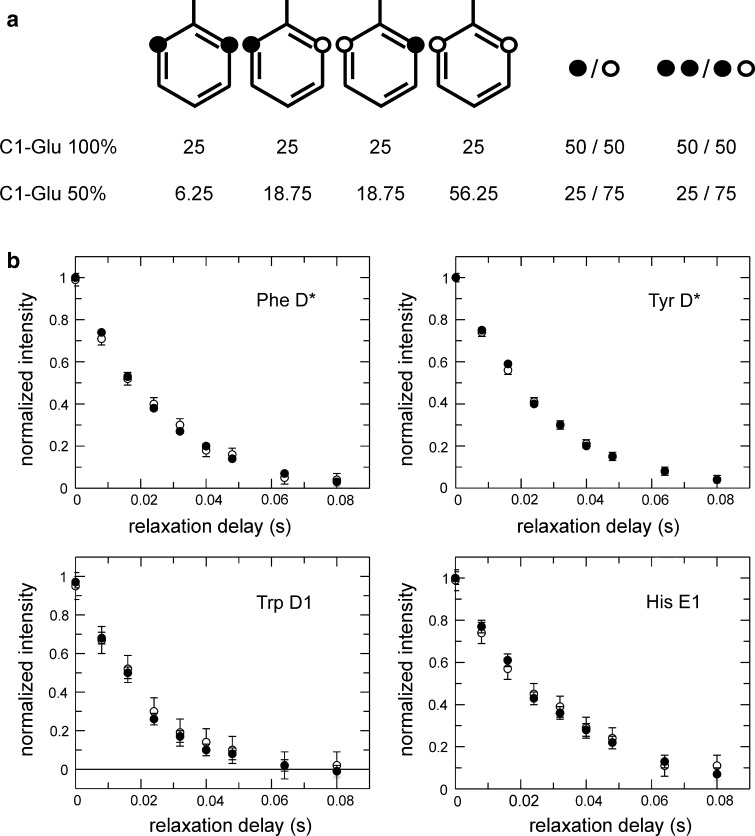
Fig. 5.
Influence of two-bond 13C–13C J couplings on measured R 2 relaxation decays. a 13C incorporation pattern in a Phe side chain resulting from labeling with 1-13C1-glucose. Black circles represent 13C-labeled positions while open circles represent 12C. The symbols to the right of the aromatic rings show the net percentages of labeled or unlabeled sites (labeled/unlabeled), and the percentages of 13C sites with or without a two-bond neighbor (labeled–labeled/labeled–unlabeled). Labeling using 100 % 1-13C1-glucose yields 50 % 13C incorporation in the Cδ positions, and 50 % of these 13Cδ have a 13C-labeled two-bond neighbour. Labeling using 50 % 1-13C1-glucose + 50 % 12C6-glucose reduces the relative number of labeled carbons, as well as that of labeled two-bond neighbours, to 25 %. b Representative R 2 relaxation decays for different aromatic side chains in samples labeled using 100 % 1-13C1-glucose (filled circles) or 50 % 1-13C1-glucose + 50 % 12C6-glucose (open circles)