Figure 1.
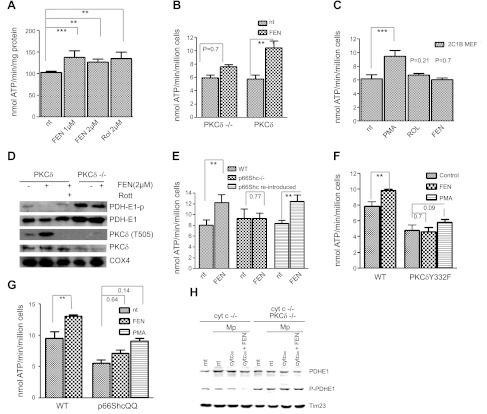
Fenretinide as coactivator of PKCδ. A) Capacity of fenretinide to accelerate the ATP synthase rate of WT MEFs equals that of retinol. WT MEFs were treated for 30 min with either 1 or 2 μM fenretinide (FEN) or 2 μM retinol (Rol), or left untreated. Pyruvate/malate-driven rates of ATP synthesis were measured as described in Materials and Methods and expressed as nanomoles ATP per minute per milligram protein or per million cells. ATP synthesis increased by 28, 25, or 28%, respectively (n=6). One of 3 independent experiments is shown. **P < 0.01, ***P < 0.001. B) Failure to stimulate ATP synthesis in PKCδ−/− MEFs indicates dependence on PKCδ; reintroduction of PKCδ restores responsiveness to fenretinide. PKCδ−/− MEFs, and PKCδ−/− MEFs reconstituted with full-length PKCδ by transfection, were treated with fenretinide and analyzed for ATP production as in A. The response of PKCδ−/− MEFs was statistically insignificant, whereas reintroduction of PKCδ reestablished response to fenretinide (45% increase, n=3). One of 4 independent experiments is shown. **P < 0.01. C) Expression of a mutant PKCδ gene deficient in retinoid-binding sites fails to rescue the PKCδ phenotype. This block is bypassed by phorbol ester (PMA). PKCδ was converted to a retinol-nonbinding form by genetic exchange of both endogenous zinc-finger domains for the PKCα C1B domain. The reengineered full-length gene was expressed in PKCδ−/− MEFs. Responsiveness to retinoids (2 μM), or PMA (100 nM) was determined as described in A. Neither retinol nor fenretinide elicited a cofactor response commensurate with WT PKCδ responses shown in A and B. A 35% increase in ATP synthesis (n=4) was observed with PMA, which bypasses the requirement for retinoid cofactor. ***P < 0.001. D) Fenretinide stimulates autophosphorylation of threonine-505 of PKCδ, indicating conversion to active enzyme. The E1 subunit of PDH is dephosphorylated, indicating PDH activation. WT and PKCδ−/− MEFs were stimulated with 2 μM fenretinide as in A. Mitochondrial proteins were separated by SDS-PAGE and analyzed by immunoblotting for the proteins indicated. Phosphorylation of T 505 of PKCδ and dephosphorylation of PDH E1 indicated activation of both enzymes, and this was prevented by rottlerin (Rott). PDH was not dephosphorylated in PKCδ−/− MEFs. One of 3 independent experiments is shown. Direct immunoblotting of PKCδ stained a contaminating band. This was eliminated by prior enrichment of PKCδ with immunoprecipitation (not shown). E) Fenretinide-mediated up-regulation of oxidative phosphorylation is ablated by p66Shc gene knockout, and it is restored by reintroduction of the intact gene. WT MEFs, MEFs with a defective p66Shc gene, and p66Shc−/−MEFs reconstituted with intact p66Shc gene were treated with fenretinide and analyzed as in A. ATP synthesis increased by 30% in WT MEFs, was not enhanced in the knockout MEFs, but was enhanced by 30% after reintroduction of p66Shc (P < 0.002 for both responders, n=4). One of 3 independent experiments is shown. **P < 0.01. F) Fenretinide-mediated up-regulation of oxidative phosphorylation is abolished in MEFs expressing the Y332F mutant PKCδ, defective in its ability to bind p66Shc. WT MEFs and PKCδ−/− MEFs reconstituted with the mutated PKCδ Y332F gene were treated with 2 μM fenretinide and analyzed as in A. WT cells responded by a 25% increase in ATP synthesis (P < 0.01, n=4), but MEFs carrying the defective PKCδ did not. The block was partially overridden by stimulation with PMA. One of 6 independent experiments is shown. **P < 0.01. G) Fenretinide-mediated up-regulation of oxidative phosphorylation is abolished in MEFs expressing the E132Q/E133Q mutant of p66Shc incapable of binding cytochrome c. WT MEFs and p66Shc−/− MEFs reconstituted with the mutated p66ShcQQ gene were treated with 2 μM fenretinide and analyzed as in A. WT cells responded by a 25% increase in ATP synthesis (P < 0.01), but responses of MEFs carrying the mutated QQ gene were attenuated. PMA partially overrode this block (35% increase, P < 0.14). One of 6 independent experiments is shown. **P < 0.01. H) PKCδ signaling is defective in mitoplasts deprived of cytochome c, and is restored by introduction of oxidized cytochrome c protein in combination with fenretinide (left panel); mitoplasts of PKCδ−/− MEFs fail to respond to cytochrome c and fenretinide (right panel). Mitochondria (mt) and mitoplasts (Mp) of cytochrome c-knockdown (cyt c−/−) MEFs or cytochrome c-knockdown/PKCδ-deficient (cyt c−/− PKCδ−/−) cells were prepared as described in Materials and Methods. Mitoplasts were reconstituted with 25 mM oxidized cytochrome c (cyt cox) with and without fenretinide for 10 min at 37°C, or left untreated (nt). Mitochondrial proteins were separated by SDS-PAGE and analyzed by immunoblot for PDH E1 content and phosphorylation status. Time 23 was used as a loading control. Dephosphorylation was observed solely in mitoplasts treated with the combination of cytochrome c and fenretinide (6-fold reduction by densitometry). All other preparations, including the mitochondria and mitoplasts devoid of PKCδ, did not yield dephosphorylated PDH E1 species. One of two repeat experiments is shown.