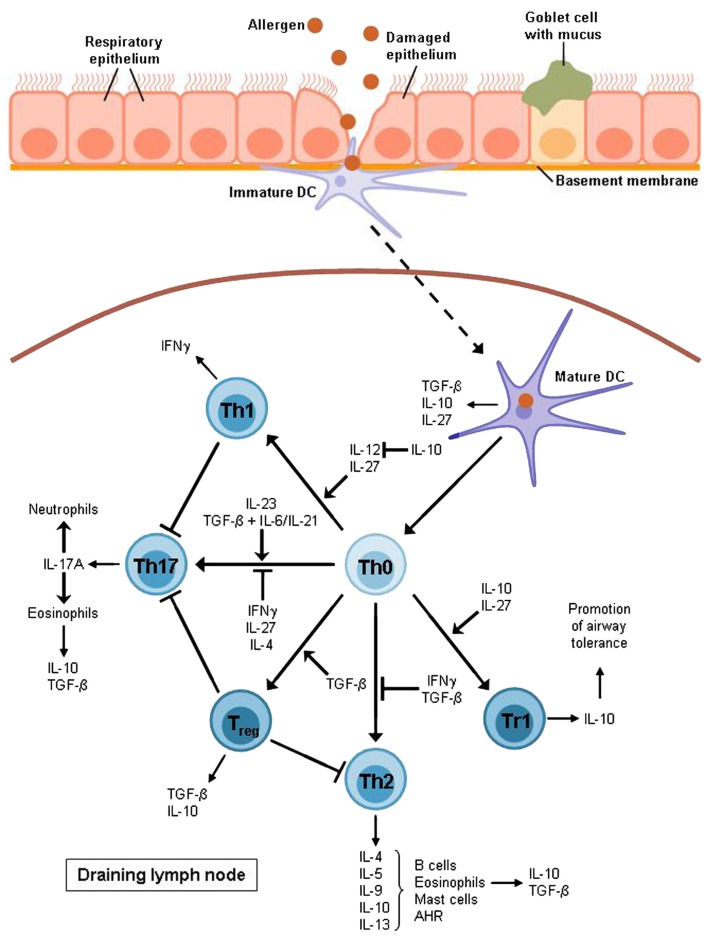
Figure 2.
T cell differentiation in allergic asthma. After allergen uptake the DC migrates to the lymph node where it activates naïve T cells to develop into different effector subsets. Allergic asthma has been found to be characterized mostly by Th2 cell expansion and a lack of Treg cells. Th2 cytokines raise the production of allergen-specific immunoglobulin E (IL-4), support the growth of eosinophils (IL-5) and mast cells (IL-9) and directly cause airway hyperreactivity (AHR; IL-13). Furthermore, Th2 cells produce IL-10 which is also expressed by eosinophils, DCs, and Treg cells, in particular Tr1 cells which also require this cytokine for differentiation. Non-asthmatic airways show a balance between Th1 and Th2 cells as IL-10 is able to suppress the differentiation of the Th1 subset by blocking IL-12 synthesis whereas the Th1 cytokine IFNγ contributes to the inhibition of Th2 development. However, this balance is impaired in allergic asthma. The Th1 subset is also supposed to have an inhibitory function on Th17 cells which produce mainly IL-17A leading to the recruitment of eosinophils and neutrophils to the airways. The development of Th17 cells depends on IL-23 or TGF-β combined with either IL-6 or IL-21.TGF-β is produced by eosinophils, DCs, and Treg cells whereas it is also required for the induction of Treg cells and for the inhibition of the Th2 subset. Apart from IL-10 and TGF-β, DCs also produce IL-27, an inhibitor of Th17 cells and an inducer of Tr1 cell development. Except for DCs, this Treg subset is also supposed to be involved in the induction of airway tolerance.