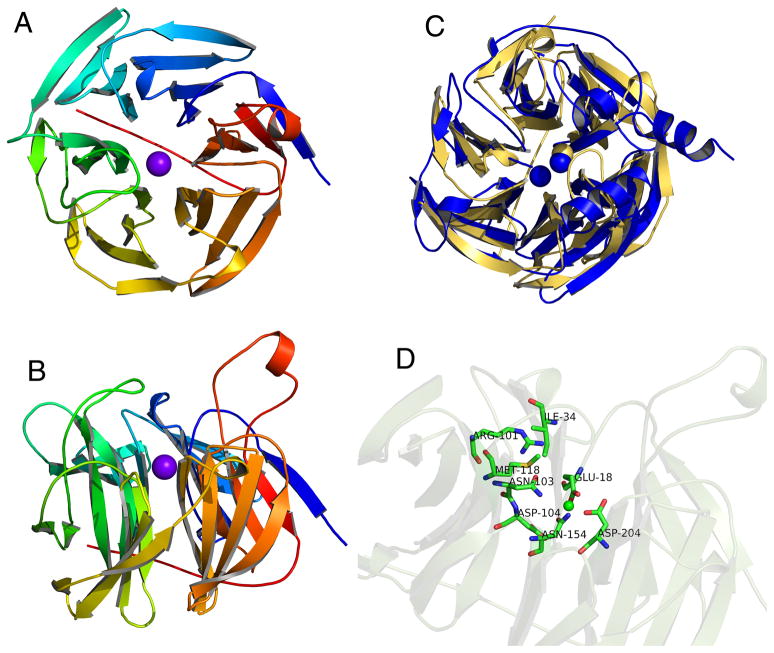
Figure 4.
Ca2+-bound crystal structure of human SMP30. A) A top view of SMP30 shows the six-bladed β-propeller fold. The C-terminal tail traverses the central tunnel of the propeller and the bound Ca2+ ion is shown in purple. B) A side view of SMP30 shows several loops that extend above the propeller near the active site which is located just above the bound Ca2+ ion. C) Overlay of the crystal structures of human SMP30 (gold) and PON1 (blue). Although both proteins have a six-bladed β-propeller fold, PON1 binds two Ca2+ ions whereas SMP30 only binds one. Furthermore, PON1 is specific for Ca2+, while SMP30 can bind several different divalent cations. D) Side view of SMP30 with the metal-binding and putative active site residues highlighted. E18, D204, and N154 are bound to the metal ion. N103 and D104 are not close enough to bind the metal. Other putative active site residues, which are located just above the metal binding site include, I34, R101, and M118.