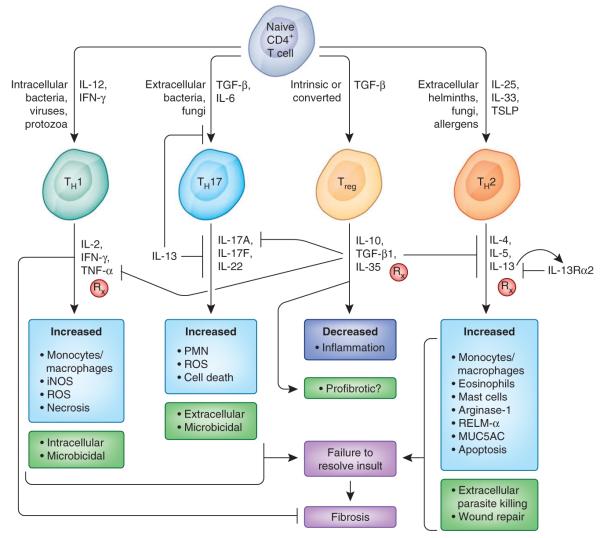
Figure 3.
Adaptive immune pathways in fibrosis. Naive CD4+ T cells differentiate into various distinct functional lineages driven by cues produced by injured epithelial cells and activated antigen presenting cells (dendritic cells and macrophages). Intracellular infections trigger IL-12–driven TH1 responses that produce IFN-γ, which activates microbicidal and cytotoxic activities that aid in pathogen clearance. Extracellular bacteria and certain fungi can lead to inflammasome activation and IL-6 production and can, in the presence of TGF-β1, drive TH17 differentiation. IL-17 from TH17 cells helps recruit neutrophils to clear the infection and exacerbates inflammation. Infection with extracellular, tissue-dwelling helminth parasites drives TH2 differentiation, with IL-4, IL-25, IL-33 and thymic stromal lymphopoietin (TSLP) from innate and epithelial sources guiding the differentiation of CD4+ TH2 cells. IL-13, when not competed for by the higher-affinity decoy receptor IL-13Rα2, binds its signaling receptor IL-13Rα1, leading to alternative activation of macrophages as well as epithelial apoptosis and myofibroblast activation. Treg cells are crucial in limiting the magnitude of TH cell responses and thereby ensure proper regulation of the wound-healing response. There is also a great deal of cross-regulation among TH cell subsets. For example, IL-13 suppresses TH17 differentiation, whereas IFN-γ can suppress IL-13–induced fibrosis by inducing classical macrophage activation and suppressing IL-13 and TGF-β1–induced collagen synthesis in myofibroblasts. Rx, pathways being targeted for therapy, either preclinically or in clinical trials; iNOS, inducible nitric oxide synthase; ROS, reactive oxygen species; PMN, polymorphonuclear leukocyte; MUC5AC, mucin-5AC.