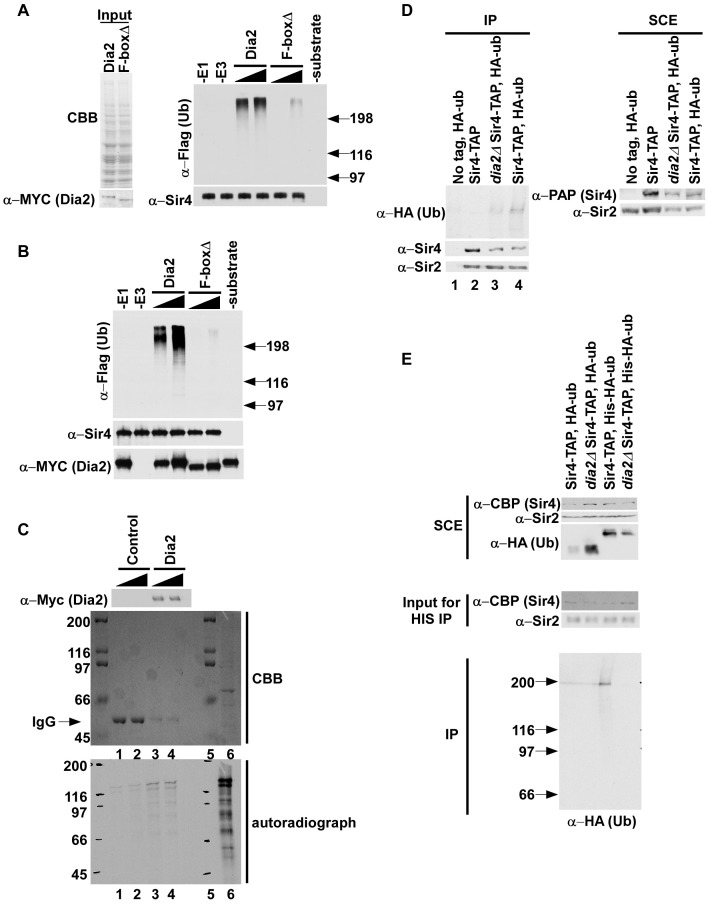
Figure 6. SCFDia2 ubiquitylates Sir4 in vitro and in vivo.
(A–B) Dia2 ubiquitylates Sir4 in vitro (A) (left panel) Cell extracts prepared from full length MYC-Dia2 or MYC-Dia2 F-boxΔ (F-boxΔ) cells were normalized using Coomassie staining (CBB, upper panel) and Western blot analysis using antibodies against the MYC epitope (bottom panel). (right panel) Two different amounts of extract were used for in vitro ubiquitylation assays using Sir4-TAP purified from dia2Δ cells as substrate, Flag-ubiquitin (Flag-ub), E1, and E2 (Cdc34). Following the ubiquitylation reaction, Sir4-CBP proteins were purified using calmodulin beads, and ubiquitylated species were detected via Western blot using Flag antibody. Sir4 were detected by Western blot using antibody against Sir4. (B) Ubiquitylation reactions were carried out as described in A except purified MYC-Dia2 or MYC-Dia2 F-boxΔ was used as E3. (C) Sir4 binds the SCFDia2 complex in vitro. Binding studies were performed using control beads, prepared by incubating beads with antibodies against the Myc epitope, or beads containing the MYC-Dia2 complex prepared as in B and two different amounts of in vitro translated 35S-methione labeled Sir4 (lane 6, 35S-Sir4 input). The presence of the SCFDia2 in binding samples was detected by Western blot (upper panel) using antibodies against the Myc epitope. In addition, binding samples were resolved by SDS-PAGE, stained with Coomassie (CBB, middle panel) and bound 35S-Sir4 was detected via autoradiography (lower panel); Lane 5 is a protein marker. (D–E) Sir4 is ubiquitylated in vivo, and this ubiquitylation is decreased in dia2Δ cells. (D) Sir4-TAP was purified from cells of the indicated genotype either with or without expression of HA-ubiquitin (HA-ub) using tandem affinity purification. Ubiquitylated species were detected using antibodies against the HA epitope. Right panel: SCE, soluble cell extract. (E). Sir4-TAP was purified from wild-type or dia2Δ mutant cells expressing either HA-ubiquitin or His-HA-Ubiquitin using IgG sepharose. After cleavage with TEV protease, the eluted proteins were denatured and purified using Ni-NTA beads. Purified ubiquitylated species were detected by Western blot using antibodies against HA.