Abstract
Rationale: Sleep-disordered breathing (SDB) has been associated with total and cardiovascular mortality, but an association with cancer mortality has not been studied. Results from in vitro and animal studies suggest that intermittent hypoxia promotes cancer tumor growth.
Objectives: The goal of the present study was to examine whether SDB is associated with cancer mortality in a community-based sample.
Methods: We used 22-year mortality follow-up data from the Wisconsin Sleep Cohort sample (n = 1,522). SDB was assessed at baseline with full polysomnography. SDB was categorized using the apnea-hypopnea index (AHI) and the hypoxemia index (percent sleep time below 90% oxyhemoglobin saturation). The hazards of cancer mortality across levels of SDB severity were compared using crude and multivariate analyses.
Measurements and Main Results: Adjusting for age, sex, body mass index, and smoking, SDB was associated with total and cancer mortality in a dose–response fashion. Compared with normal subjects, the adjusted relative hazards of cancer mortality were 1.1 (95% confidence interval [CI], 0.5–2.7) for mild SDB (AHI, 5–14.9), 2.0 (95% CI, 0.7–5.5) for moderate SDB (AHI, 15–29.9), and 4.8 (95% CI, 1.7–13.2) for severe SDB (AHI ≥ 30) (P-trend = 0.0052). For categories of increasing severity of the hypoxemia index, the corresponding relative hazards were 1.6 (95% CI, 0.6–4.4), 2.9 (95% CI, 0.9–9.8), and 8.6 (95% CI, 2.6–28.7).
Conclusions: Our study suggests that baseline SDB is associated with increased cancer mortality in a community-based sample. Future studies that replicate our findings and look at the association between sleep apnea and survival after cancer diagnosis are needed.
Keywords: cancer, cohort study, mortality, obstructive sleep apnea, sleep-disordered breathing
At a Glance Commentary
Scientific Knowledge on the Subject
Results from in vitro and animal studies suggest that intermittent hypoxia promotes cancer tumor growth, but there are no previous studies in humans on the association between sleep-disordered breathing (SDB) and cancer incidence or mortality.
What This Study Adds to the Field
This population-based epidemiologic study suggests a strong and dose–response association between SDB and cancer mortality.
Sleep disordered breathing (SDB) is characterized by recurrent episodes of total or partial obstruction of the upper airway (apnea or hypopnea) during sleep that are associated with intermittent hypoxemia, repeated sleep disruption, and snoring (1). The prevalence of moderate to severe SDB has been estimated to be as high as 6% among U.S. adults and rising as the prevalence of obesity, a major risk factor for SDB, continues to increase (2–4). SDB is associated with a variety of psychopathological disorders (depression, reduced quality of life) and increased risk of occupational and motor vehicle injuries, as well as with a variety of cardiovascular disease outcomes, hypertension, and the metabolic syndrome (3, 5, 6). These associations have been explained by the profound metabolic and sympathetic system disruption associated with repeated hypoxemic events (6, 7).
Chronic hypoxia, a common feature in solid tumor tissue, has been associated with therapeutic resistance, tumor progression, and metastatic potential (8–10). In vitro studies have further demonstrated that cultured lung cancer cells subject to intermittent hypoxia are more resistant to radiation and apoptosis and are more prone to metastasis (11).
Recent results from a study using a mouse model for obstructive sleep apnea showed that intermittent hypoxia was associated with accelerated cancer progression (12). These effects might be mediated by the hypoxia-induced increased tumor tissue angiogenesis and resulting cell proliferation and tumor growth (13–15).
To our knowledge, no epidemiologic studies to date have explored the possible association between SDB and cancer incidence or mortality in a population sample.
In a community-based cohort we have demonstrated an association between SDB and both total and cardiovascular mortality (16). We used updated data from this cohort to test the hypothesis that SDB is associated with increased cancer mortality.
Methods
As described in detail elsewhere (2, 16), the Wisconsin Sleep Cohort was established in 1988 as a prospective community-based study of predictors and natural history of sleep disorders. In brief, 30- to 60-year-old men and women living in south-central Wisconsin were selected from payroll records of several Wisconsin state agencies with job titles ranging from unskilled to professional. Of the 2,940 individuals invited to undergo an overnight in-laboratory protocol, 1,546 (53%) agreed to participate and were successfully studied. Compared with the entire sampling frame, cohort participants had a slightly healthier profile (2, 17) and lower death rate (16).
Data Variables
Consenting participants underwent a baseline overnight 18-channel polysomnography (PSG) (Grass model 78; Quincy, MA) at the University of Wisconsin General Clinical Research Center using a standard protocol (2). The PSG recorded sleep state using electroencephalography, electrooculography, and electromyography; breathing, using respiratory inductance plethysmography (Respitrace; Ambulatory Monitoring, Ardsley, NY) and nasal and oral airflow (ProTec thermocouples; Hendersonville, TN); and oxyhemoglobin saturation, using pulse oximetry (Ohmeda Biox 3740; Englewood, CO). Each 30-second epoch of the polysomnographic recordings was scored for sleep stage and apnea and hypopnea events by trained technicians and reviewed using standard criteria (18). Apnea was defined as cessation of nasal and oral airflow for 10 seconds or more and hypopnea as a discernible reduction in breathing (sum of chest and abdominal excursions) with a reduction in oxyhemoglobin saturation of 4% or greater.
The apnea-hypopnea index (AHI) was calculated as the mean number of apnea and hypopnea events per hour of sleep. As in our previous analyses of these data (16), AHI was categorized according to widely used cutpoints in the literature and clinical practice: normal (AHI < 5), mild SDB (AHI 5–14.9), moderate SDB (AHI 15–29.9), and severe SDB (AHI ≥ 30). In the present analyses, participants who, by their request, used their continuous positive airway pressure (CPAP) device during the baseline overnight PSG study (n = 9) were also included in the severe SDB category; this inclusion was based on the assumption that CPAP users have had severe SDB in the more or less recent past and that our hypothesis relates to chronic effects of intermittent hypoxia on cancer growth and progression.
To examine the association of cancer mortality with oxygen saturation, we used a subset of 1,306 participants with event-by-event saturation data (representing 33 cancer deaths) from which the hypoxemia index (percent sleep time below 90% oxyhemoglobin saturation) could be calculated. We defined the hypoxemia index cutpoints based on the analogous percentile distribution as the above AHI cutpoints in our study population; the AHI percentiles were: 73% of subjects with AHIs below 5 events per hour, 90% below 15 events per hour, and 97% below 30 events per hour. The corresponding percentile values for hypoxemia index were 0.8, 3.6, and 11.2%, respectively.
Standardized interviews and several objective measures and tests were performed by trained personnel. Information used for this analysis included alcohol use (number of drinks/wk), cigarette smoking (current, former, or never), educational level (less than high school or not), self-rated general health (excellent, very good, good, fair, poor), severe daytime sleepiness (a “yes” response to the question “During a typical day do you experience excessive sleepiness when it is difficult to fight an uncontrollable urge to fall asleep?”), physical activity, history of physician-diagnosed diabetes, history of physician-diagnosed sleep apnea, and use of CPAP treatment. Body mass index (BMI; weight in kilograms divided by height in meters squared), waist circumference, and age (yr) at baseline were used as continuous variables.
Deaths in the cohort occurring up to November 11, 2011 were identified by matching social security numbers with two death record sources: the National Death Index (NDI) and the Wisconsin State Bureau of Health Information and Policy, Vital Records Section. Matches on social security number were verified with participants’ age and sex. All deaths in Wisconsin, reported by the vital records of Wisconsin, were also identified in the NDI; in addition, four deaths occurring outside of Wisconsin were identified by the NDI. Date of death was available on all decedents, and underlying and contributory causes of deaths were available from Wisconsin Vital Statistics. Wisconsin Vital Statistics and the NDI supplied files with data on each individual death, including the cause of death and corresponding description as abstracted from individual death certificates. Cancer mortality was defined as an International Classification of Diseases (ICD)-9 code 140–239 or ICD-10 codes C00-D48.
Statistical Analysis
A total of 1,522 participants have complete information on the key variables used for the present analyses. Person-years were accumulated from baseline study to date of death, loss to follow-up date, or November 11, 2011, whichever came first. We computed all-cause and cancer mortality rates (deaths/1,000 person-years) and 95% confidence intervals (CI) by SDB categories at baseline. Kaplan-Meier techniques were used to compare survival across SDB categories (19). Cox proportional hazards regression was used to estimate adjusted hazard ratios and 95% CIs (20). Because of the strong dependence of cancer risk on age, Cox regression models were based on age as the time scale, allowing for left truncation (late entry) (21). In addition to the analyses based on the AHI, we also ran hazard models to predict cancer death using the hypoxemia index (both as a continuous and as a categorical variable, as defined above) in the 1,306 participants for whom this information was available.
Models were examined for sex, linear and quadratic terms for BMI, smoking, alcohol use, physical activity, and educational status as potential confounding factors. Statistical trends were examined by testing the significance of SDB (AHI or hypoxemia index categories) as a linear term. Diagnostic and residual plots were examined to test the proportional hazards assumptions. All statistical analyses were conducted using SAS software (Version 9.1.3; SAS Institute, Cary, NC).
Analyses were repeated after excluding participants who reported use of CPAP at any point during the study follow-up period (n = 151). In addition, sensitivity analysis including only deaths from solid cancers as the outcome (i.e., considering the three deaths attributed to lymphoproliferative malignancies as censored observations) was also conducted.
Results
Among the 1,522 participants, 365 (24%) had at least mild SDB and 59 (4%) had severe apnea (AHI ≥ 30 or wore CPAP during the sleep assessment) (Table 1). Participants with increasing severity of SDB had significantly higher mean BMI, were more frequently male, lower educated, self-rated fair or poor health, and reported severe daytime sleepiness. There were no clear or consistent differences across SDB categories on age, alcohol consumption, or smoking.
TABLE 1.
CHARACTERISTICS OF THE STUDY POPULATION AND DEATH RATES ACCORDING TO SLEEP-DISORDERED BREATHING CATEGORIES
| Sleep-disordered Breathing (AHI Range) |
|||||
| Absent | Mild | Moderate | Severe | ||
| All | (<5) | (5–14.9) | (15–29.9) | (≥30)* | |
| No. | 1,522 | 1,157 | 222 | 84 | 59 |
| Age, mean (SD), yr | 47.5 (8.1) | 46.8 (7.9) | 49.8 (8.1) | 50.8 (8.6) | 49.4 (8.6) |
| BMI, mean (SD), kg/m2 | 29.9 (6.6) | 28.7 (5.9) | 32.3 (6.5) | 34.3 (7.1) | 38.6 (8.4) |
| Alcoholic drinks/wk, mean (SD) | 3.9 (6.2) | 3.8 (6.1) | 4.1 (6.7) | 4.7 (6.4) | 4.4 (7.3) |
| Male sex, % | 55.1 | 50.8 | 64.9 | 72.6 | 78.0 |
| Education (≤ high school), % | 25.7 | 24.9 | 30.6 | 31.0 | 30.5 |
| Smoking, % | |||||
| Current | 18.1 | 18.5 | 16.7 | 15.5 | 18.6 |
| Former | 38.6 | 35.1 | 41.9 | 51.2 | 50.9 |
| Self-rated fair/poor health, % | 4.3 | 3.4 | 3.3 | 8.9 | 19.7 |
| Severe daytime sleepiness, % | 24.5 | 23.1 | 24.9 | 32.5 | 39.7 |
| Mortality rate per 1,000 person-yr (95% CI) | |||||
| All causes | 4.29 (3.54–5.17) | 3.24 (2.50–4.12) | 6.88 (4.45–10.2) | 5.02 (2.02–10.3) | 15.57 (8.72–25.7) |
| Cancer | 1.92 (1.42–2.53) | 1.54 (1.05–2.19) | 1.92 (0.77–3.97) | 3.58 (1.16–8,36) | 7.27 (2.92–15.0) |
Definition of abbreviations: AHI = apnea-hypopnea index; CI = confidence interval.
Includes participants who were subject to CPAP treatment during the polysomnography study (n = 9).
There were a total of 112 deaths in this cohort; 50 of these deaths were attributed to cancer. The most frequent cancer death was lung (n = 8), followed by colorectal, ovary and endometrial (4 each), brain, breast, bladder, and liver (3 each), and other cancer sites with 1 to 2 deaths each.
Both total and cancer mortality increased linearly and significantly with increasing SDB severity (Table 1). Of the 50 cancer deaths, 31 occurred in the participants without SDB, 7 in those with mild SDB, 5 in the moderate SDB group, and 7 in the severe SDB group. The corresponding rates and 95% CIs are shown in Table 1. Compared with those without SDB, participants in the severe SDB category had a crude relative risk of mortality of all causes and of cancer mortality of 3.6 and 3.8, respectively (both P < 0.001).
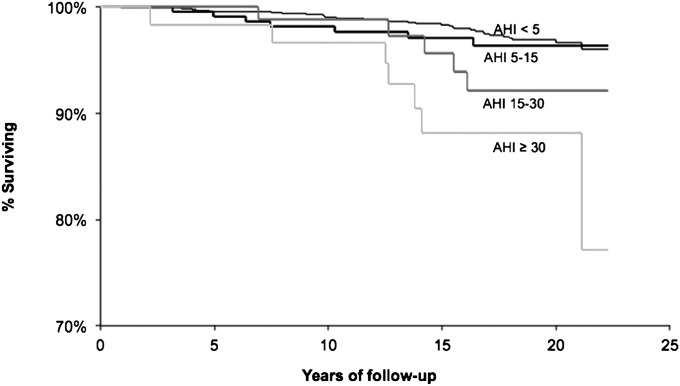
Kaplan-Meier analysis shows a dose–response decrease in overall survival free of cancer with the presence and increased severity of SDB (Figure 1). Adjusted relative hazards of mortality associated with SDB categories are shown in Table 2. After adjusting for age, sex, BMI, and smoking, a significant trend of increasing hazards of mortality with increasing SDB severity was observed for both mortality of all causes (P-trend = 0.001) and for cancer mortality (P-trend = 0.005). Additional adjustment for physical activity, alcohol use, education, diabetes, waist circumference, or sleep duration did not materially change these results (not shown). Excluding nonsolid cancers (n = 3), the relative hazard estimates were virtually identical to those shown in Table 2 (results not shown).
Figure 1.
Survival free of cancer mortality according to categories of sleep-disordered breathing, Wisconsin Sleep Cohort, 1989–2011. AHI = apnea-hypopnea index.
TABLE 2.
ADJUSTED RELATIVE HAZARDS OF TOTAL AND CANCER MORTALITY ACCORDING TO SLEEP-DISORDERED BREATHING CATEGORIES
| SDB (AHI Range) | All-Cause Mortality | Cancer Mortality |
| Absent (<5) | 1.0 | 1.0 |
| Mild SDB (5–14.9) | 1.8 (1.1–2.8) | 1.1 (0.5–2.7) |
| Moderate SDB (15–29.9) | 1.1 (0.5–2.5) | 2.0 (0.7–5.5) |
| Severe SDB (≥30)* | 3.4 (1.7–6.7) | 4.8 (1.7–13.2) |
| P for trend | 0.0014 | 0.0052 |
Definition of abbreviations: AHI = apnea-hypopnea index; BMI = body mass index; CI = confidence interval; CPAP = continuous positive airway pressure; SDB = sleep-disordered breathing.
Data are presented as adjusted relative hazard (95% CI). Adjusted for age (time scale), sex, BMI, BMI2, and smoking.
Includes participants who were subject to CPAP treatment during the polysomnography study (n = 9).
Among study participants for whom this information was available, the hypoxemia index was strongly associated with cancer mortality in a dose–response fashion (P for trend = 0.0008) (Table 3); the adjusted hazard in participants in the top hypoxemia index category was more than eight times higher than that in the lower category. When used as a continuous variable, an increase in one log-unit in the hypoxemia index was associated with an adjusted hazard ratio of cancer mortality of 1.9 (95% CI, 1.3–2.9; P = 0.002).
TABLE 3.
ADJUSTED RELATIVE HAZARDS OF CANCER MORTALITY ACCORDING TO HYPOXEMIA INDEX
| Hypoxemia Index* | Relative Hazards of Cancer Mortality (95% CI) |
| Percentile < 73 (<0.8% of the time) | 1.0 |
| Percentile 73–89 (0.8–3.6% of the time) | 1.6 (0.6–4.4) |
| Percentile 90–97 (3.6–11.2% of the time) | 2.9 (0.9–9.8) |
| Percentile > 97 (>11.2% of the time) | 8.6 (2.6–28.7) |
| P for trend | 0.0008 |
Definition of abbreviations: AHI = apnea-hypopnea index; BMI = body mass index; CI = confidence interval.
Adjusted for age (time scale), sex, BMI, BMI2, and smoking. Hypoxemia index = percent sleep time below 90% oxyhemoglobin saturation; analyses based on a subset of 1,306 participants on whom O2 saturation data were available.
Cut-offs defined according to the same percentile distribution as that resulting from the standard AHI cut-offs used in this study (AHI = 5, 15, and 30).
In stratified analyses, a dose–response association between SDB and cancer mortality was more evident and more clearly statistically significant among nonobese compared with obese participants and among participants without perceived daytime sleepiness compared with participants with perceived daytime sleepiness (Table 4). However, formal tests for interaction according to obesity and sleepiness were both nonsignificant (both P > 0.1). Similarly, no evidence of statistically significant interaction was found in stratified analyses according to sex or age (not shown).
TABLE 4.
ADJUSTED RELATIVE HAZARDS OF CANCER MORTALITY ACCORDING TO SLEEP-DISORDERED BREATHING CATEGORIES STRATIFIED ACCORDING TO OBESITY STATUS AND PRESENCE OF SEVERE DAYTIME SLEEPINESS
| Obesity Present |
Sleepiness Present |
|||
| SDB (AHI Range) | Yes | No | Yes | No |
| Absent (<5) | 1.0 | 1.0 | 1.0 | 1.0 |
| Mild SDB (5–14.9) | 1.1 | 1.1 | 0.6 | 1.5 |
| Moderate SDB (15–29.9) | 0.5 | 4.5† | 1.0 | 2.8 |
| Severe SDB (≥30)* | 4.1† | 5.2 | 3.9 | 4.9† |
| P for trend | 0.085 | 0.0171 | 0.19 | 0.0124 |
Definition of abbreviations: AHI = apnea-hypopnea index; CPAP = continuous positive airway pressure; SDB = sleep-disordered breathing.
Adjusted for age (time scale), sex, BMI, BMI2, and smoking.
Includes participants who were subject to CPAP treatment during the polysomnography study (n = 9).
P < 0.05.
When participants who used CPAP during follow-up (n = 151) were excluded from the analysis, the association of untreated SDB and cancer mortality were slightly stronger than those shown in Table 2 (relative hazards of cancer mortality for increasing severity of untreated SDB categories of 1.2, 2.7, and 5.0, respectively; P-trend = 0.005).
Discussion
To our knowledge, this is the first population-based cohort study documenting an association between baseline SDB and cancer mortality over a follow-up period lasting up to 22 years. Remarkably, the association was stronger in relative terms than that of SDB with mortality from all causes (Table 2) as well as that previously observed for cardiovascular mortality in this cohort (16) and in other studies (22–26).
The association remained significant after adjusting for possible confounding variables including age, sex, smoking, BMI, physical activity, diabetes, waist circumference, and sleep duration. The association remained evident when restricted to solid cancers only and persisted when patients treated with CPAP were excluded from the analyses.
Our decision to characterize SDB based on the baseline rather than in the most recent time-dependent values was made a priori with the goal of minimizing the likelihood of reverse causation bias. Because of a potentially long and protracted natural history, cancer patients may lose weight years before their death, which in turn might result on an improvement of SDB, thus making a more recent measurement less relevant than earlier SDB assessments.
Because this is the first study documenting the association of SDB and cancer mortality, replication of these findings in other populations and settings is critical. However, our observations are consistent with our a priori hypothesis, grounded on evidence from recent in vitro and animal studies on the effects of hypoxia in cancer progression. Cancer cells subjected to either chronic (8–10) or intermittent hypoxia (11) show increased resistance to therapy (e.g., radiation) and malignant progression. Furthermore, recent experiments in a melanoma-injected mouse model demonstrate that intermittent hypoxia mimicking sleep apnea in humans increases tumor growth (12). This association might be explained by the increased angiogenesis associated with tissue hypoxemia (13–15). Hypoxia induces overexpression of hypoxia-inducible factor (HIF-1α), which in turn triggers up-regulation of proangiogenic mediators, such as vascular endothelial growth factor, in tumor cells (15). Newly formed vascular networks, however, usually present structural and functional abnormalities that lead to reduced perfusion and oxygen delivery to the tumor tissue leading to necrosis (27), which is a predictor of aggressive cancer progression and poor prognosis (28). Consistent with the experimental evidence from the intermittent hypoxemia models, in our study the association with cancer mortality was even stronger when, instead of the AHI, SDB was characterized using the hypoxemia index (percent sleep time below 90% oxyhemoglobin saturation) in the subset of participants for whom this information was available (Table 3).
Our study is limited by the lack of data on cancer incidence in the members of our cohort. Studying the survival after cancer incidence or diagnosis would be the most appropriate study design to address the relevance of the above hypothesis to humans. However, until a cohort study of patients in whom cancer was recently diagnosed who are subjected to a PSG study and followed to mortality becomes available, our study results offer indirect evidence in support of the study hypothesis. Indeed, assuming that SDB does not affect cancer incidence, studying overall cancer mortality offers a valid surrogate for a cancer survival study.
Another limitation of our study is the relatively small number of events. However, the strong statistical significance of the results supports the robust nature of our findings. Nevertheless, our study might have had inadequate power to detect statistically significant interactions (such as those suggested by the results presented in Table 4). Furthermore, the study did not have sufficient statistical power for analysis of SDB with site-specific cancer mortality. This limitation, however, is an unlikely explanation for the strong associations observed in this study. If associations are specific for distinct cancer sites but not others, combining all cancer mortality will result in dilution of effects and thus tend to bias the results toward the null.
Strengths of our study include the population-based nature of our cohort, thus free of selection biases potentially affecting clinic-based samples of patients, the state-of-the-art methods used to assess SDB, and the virtually complete follow-up of our study participants.
In summary, this is the first study documenting an association between SDB and risk of cancer mortality in a population-based sample. Replication of these findings would be necessary, particularly in studies that examine the role of treated and untreated sleep apnea in predicting survival in patients diagnosed with cancer.
The potential implications of this finding are important. SDB is strongly associated with obesity, and it might be one of the mechanisms explaining the well-documented but not entirely understood association between obesity and cancer mortality (29). Furthermore, if this association is validated in further clinical and population-based studies, diagnosis and treatment of SDB in cancer patients may be indicated to prolong survival in cancer patients.
Supplementary Material
Acknowledgments
The authors thank Mihai Teodorescu, M.D., Amanda Rasmuson, M.S., Kathryn Pluff, Robin Stubbs, Kathy Stanback, Mary Sundstrom, Nicole Salzieder, Tricia Denman, Erika Hagen, Ph.D., Katherine Cacic, D.N.P., A.P.N.P., Eric Young, Jodi Barnet, M.S., Steven Weber, Ph.D., and Patty Grubb for their technical assistance. The study was performed at the University of Wisconsin–Madison, School of Medicine and Public Health, Madison WI.
Footnotes
Supported by National Institutes of Health grants R01HL062252 and 1UL1RR025011, and Spanish Ministry of Economy and Competiveness grant SAF2011-22576.
Author Contributions: Conception and design: F.J.N., P.E.P., T.Y., R.F. Acquisition of data or analysis and interpretation of data: F.J.N., P.E.P., T.Y., L.F., K.M.H., R.F. Drafting of manuscript or revising critically for important intellectual content: F.J.N., P.E.P., T.Y., L.F., K.M.H., R.F. Final approval of the version to be published: F.J.N., P.E.P., T.Y., L.F., K.M.H., R.F.
Originally Published in Press as DOI: 10.1164/rccm.201201-0130OC on May 20, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med 2005;142:187–197 [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–1235 [DOI] [PubMed] [Google Scholar]
- 3.IOM Committee on Sleep Medicine and Research BoHSP Sleep disorders and sleep deprivation: An unmet public health problem. Washington, DC: Institutes of Medicine; 2006 [Google Scholar]
- 4.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol 2005;99:1592–1599 [DOI] [PubMed] [Google Scholar]
- 5.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002;165:1217–1239 [DOI] [PubMed] [Google Scholar]
- 6.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008;5:136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 2010;90:47–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raghunand N, Gatenby RA, Gillies RJ. Microenvironmental and cellular consequences of altered blood flow in tumours. Br J Radiol 2003;76:S11–S22 [DOI] [PubMed] [Google Scholar]
- 9.Ahn GO, Brown M. Targeting tumors with hypoxia-activated cytotoxins. Front Biosci 2007;12:3483–3501 [DOI] [PubMed] [Google Scholar]
- 10.Wouters A, Pauwels B, Lardon F, Vermorken JB. Review: implications of in vitro research on the effect of radiotherapy and chemotherapy under hypoxic conditions. Oncologist 2007;12:690–712 [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Song X, Wang X, Wei L, Liu X, Yuan S, Lv L. Effect of chronic intermittent hypoxia on biological behavior and hypoxia-associated gene expression in lung cancer cells. J Cell Biochem 2010;111:554–563 [DOI] [PubMed] [Google Scholar]
- 12.Almendros I, Montserrat JM, Ramirez J, Torres M, Duran-Cantolla J, Navajas D, Farre R. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J 2012;39:215–217 [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998;394:485–490 [DOI] [PubMed] [Google Scholar]
- 14.Toffoli S, Michiels C. Intermittent hypoxia is a key regulator of cancer cell and endothelial cell interplay in tumours. FEBS J 2008;275:2991–3002 [DOI] [PubMed] [Google Scholar]
- 15.Harris AL. Hypoxia–a key regulatory factor in tumour growth. Nat Rev Cancer 2002;2:38–47 [DOI] [PubMed] [Google Scholar]
- 16.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin Sleep Cohort. Sleep 2008;31:1071–1078 [PMC free article] [PubMed] [Google Scholar]
- 17.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378–1384 [DOI] [PubMed] [Google Scholar]
- 18.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine task force. Sleep 1999;22:667–689 [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481 [Google Scholar]
- 20.Cox D. Regression models and life table (with discussion). J R Stat Soc, B 1972;34:187–220 [Google Scholar]
- 21.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol 1997;145:72–80 [DOI] [PubMed] [Google Scholar]
- 22.He J, Kryger MH, Zorick FJ, Conway W, Roth T. Mortality and apnea index in obstructive sleep apnea: experience in 385 male patients. Chest 1988;94:9–14 [PubMed] [Google Scholar]
- 23.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046–1053 [DOI] [PubMed] [Google Scholar]
- 24.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034–2041 [DOI] [PubMed] [Google Scholar]
- 25.Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, De la Cruz-Moron I, Perez-Ronchel J, De la Vega-Gallardo F, Fernandez-Palacin A. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest 2005;128:624–633 [DOI] [PubMed] [Google Scholar]
- 26.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009;6:e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist 2004;9:10–17 [DOI] [PubMed] [Google Scholar]
- 28.Bachmann IM, Ladstein RG, Straume O, Naumov GN, Akslen LA. Tumor necrosis is associated with increased alphavbeta3 integrin expression and poor prognosis in nodular cutaneous melanomas. BMC Cancer 2008;8:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdullah A, Wolfe R, Stoelwinder JU, de Courten M, Stevenson C, Walls HL, Peeters A. The number of years lived with obesity and the risk of all-cause and cause-specific mortality. Int J Epidemiol 2011;40:985–996 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.