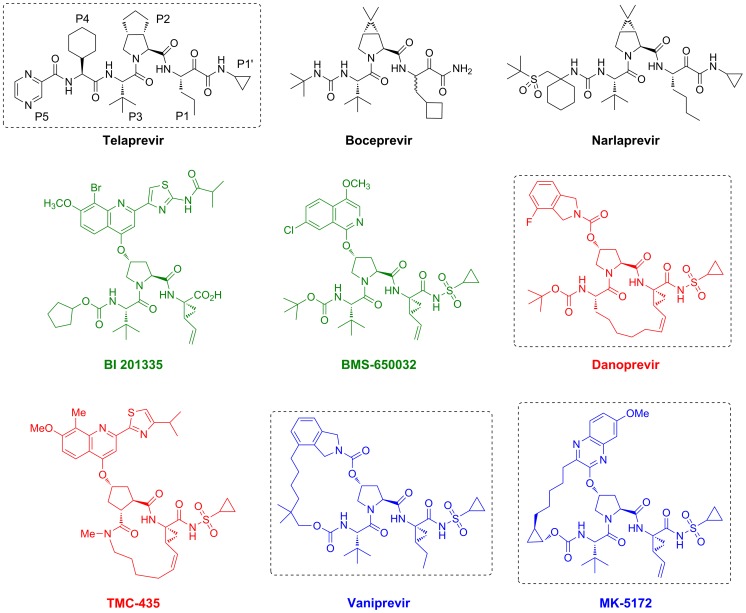
Figure 1. The chemical structures of NS3/4A protease inhibitors.
The canonical nomenclature for drug moiety positioning is indicated using telaprevir. Telaprevir (black), danoprevir (red), vaniprevir and MK-5172 (blue) are representative of many other protease inhibitors in development. Telaprevir, recently approved for clinical use, is an acyclic ketoamide inhibitor that forms a reversible, covalent bond with the protease. Danoprevir, currently in phase II clinical trials, is a non-covalent acylsulfonamide inhibitor with a P1–P3 macrocycle. Vaniprevir and MK-5172 are also non-covalent acylsulfonamide inhibitors, but contain P2–P4 macrocycles. Vaniprevir and MK-5172 differ in the construction of their P2 moieties: vaniprevir contains a carbamate linkage between the P2 proline and the isoindoline moiety, whereas MK-5172 contains a shorter ether linkage between its P2 proline and the quinoxaline moiety.