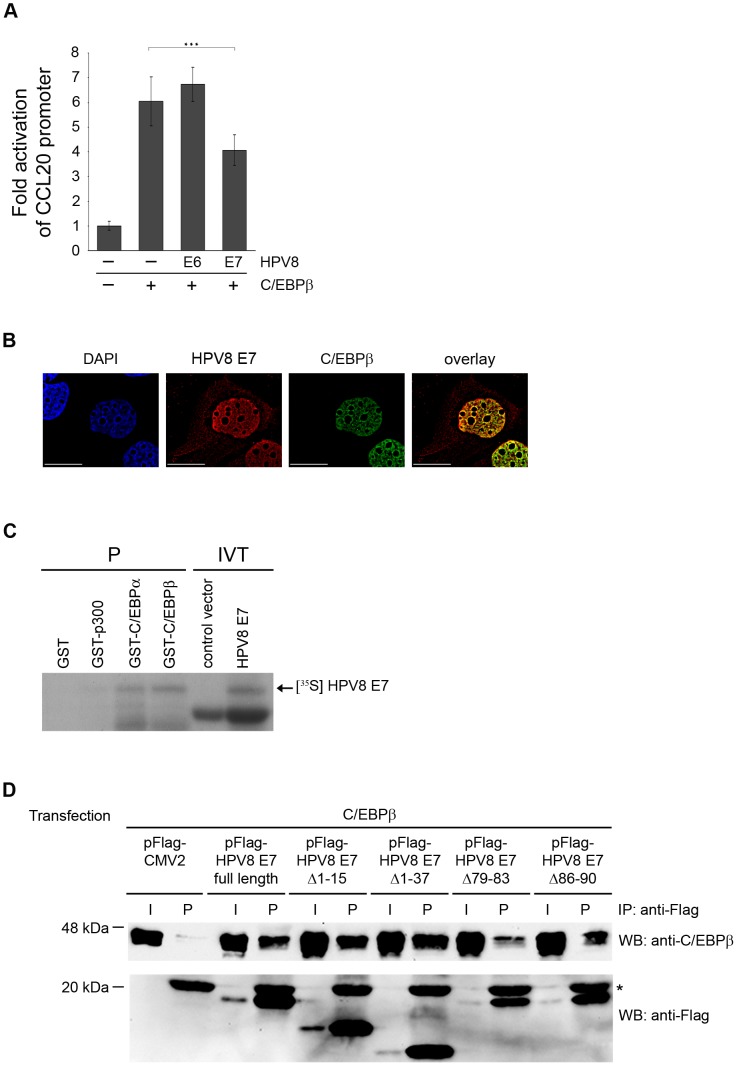
Figure 5. HPV8 E7 directly interacts with C/EBPβ and suppresses C/EBPβ-induced activation of the CCL20 promoter.
(A) NHK were transfected with CCL20 promoter luciferase construct (0.5 µg) and C/EBPβ (0.4 µg) in the presence or absence of HPV8 E6 (0.8 µg) or HPV8 E7 (0.8 µg) pcDNA3.1+ expression vectors. Total amount of DNA was adjusted with empty vector. After 24 h the luciferase activity was measured and normalized to protein concentration of the respective luciferase extract. The normalized luciferase activity of the control transfection was set at 1. Transfections were conducted in triplicates. Shown are mean values from three independent experiments ± SD. Asterisks represent statistical significance, p<0.001. (B) HPV8 E7 co-localizes with C/EBPβ in the keratinocyte nucleus. RTS3b cells seeded on glass coverslips were co-transfected with Flag-HPV8 E7 and ECFP-C/EBPβ expression vectors and stained with DAPI (blue, first panel) as well as anti-Flag antibody (red, second panel). ECFP-C/EBPβ is shown in third panel (green). Cells were analyzed by deconvolution fluorescence microscopy. The overlays and co-localization (yellow) are displayed in the fourth panel. Bars correspond to 20 µm. (C) In pull-down assays GST, GST-p300, GST-C/EBPα or GST-C/EBPβ were incubated with in vitro-translated HPV8 E7 protein (IVT, input) and precipitated (P) by glutathione Sepharose beads. pcDNA3.1+ vector (control vector) was used as a control for HPV8 E7 in vitro translation. Proteins were visualized by SDS-PAGE and autoradiography. (D) C33A cells were transfected with pFlag-CMV2, the Flag-tagged HPV8 E7 construct or deletion mutants thereof and co-transfected with the C/EBPβ expression vector. After 24 h cell lysates were prepared and precipitated with anti-Flag agarose beads (IP). The precipitates (P) and input (I, 10 µl of the lysates) were analyzed with anti-C/EBPβ or anti-Flag antibodies by Western blot (WB). The anti-Flag antibody light chain present in the precipitates is marked with a star (*).