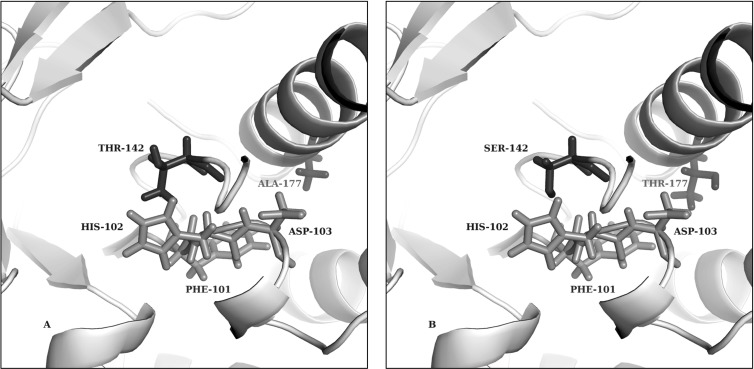
Fig 7.
Structure predictions for the xylose isomerase active site in the wild type and a mutant (xylA*3). The smaller size of SER-142 in the xylose isomerase mutant could loosen structural inhibition. In addition, THR-177 in the xylose isomerase mutant seems to open the active site, thereby allowing xylose to more easily interact with this binding pocket. Shown are the mutations near the active site of xylose isomerase in predicted three-dimensional structures of wild-type (A) and mutant (B) xylose isomerase. Shown are the mutation sites (SER-142 and THR-177) and their wild-type counterpart residues and the PHE-101, HIS-102, and ASP-103 active-site residues.