Figure 4. MYXV-based killing of human MM cells in vitro requires virion binding.
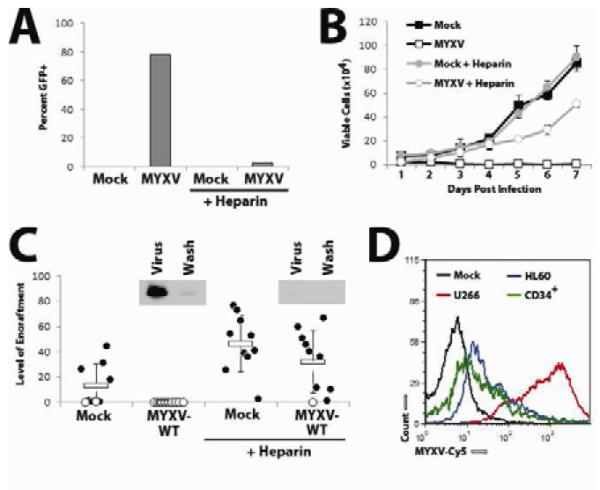
(A) U266 cells were infected with vMyx-GFP at MOI=10 in either the presence of absence of 5% soluble heparin. 24 hours later, the percent of cells expressing GFP was quantified using flow cytometry. (B) U266 cells were infected with vMyx-GFP at MOI=10 in either the presence of absence of 5% soluble heparin and the number of trypan blue excluding cells was determined at the indicated times using a hemocytometer. (C) U266 cells were mock-treated or treated with vMyx-GFP in either the presence or absence of 5% soluble heparin and then injected IV into NSG mice. Six weeks after injection, bone marrow was harvested from each mouse, stained with antibodies against human HLA-A,B,C and analyzed using flow cytometry. Data is presented as ‘level of engraftment’ which corresponds to the percent of HLA-A,B,C+ cells in the bone marrow of each mouse. Mice displaying any level of HLA-A,B,C+ cells were scored as engrafted (●) while mice without any detectable HLA-A,B,C+ cells were scored as non-engrafted (○). Inset depicts the binding of MYXV virions in the presence or absence of soluble heparin as detected by immunoblot using an αMYXV polyclonal antiserum. (D) U266 cells (red), HL60 cells (blue), or normal CD34+ stem cells (green) were incubated with vMYXV-Cy5 for one hour and then washed extensively. Cy5 florescence (indicating virion binding) was then analyzed using flow cytometry and compared to mock-treated cells (black).
