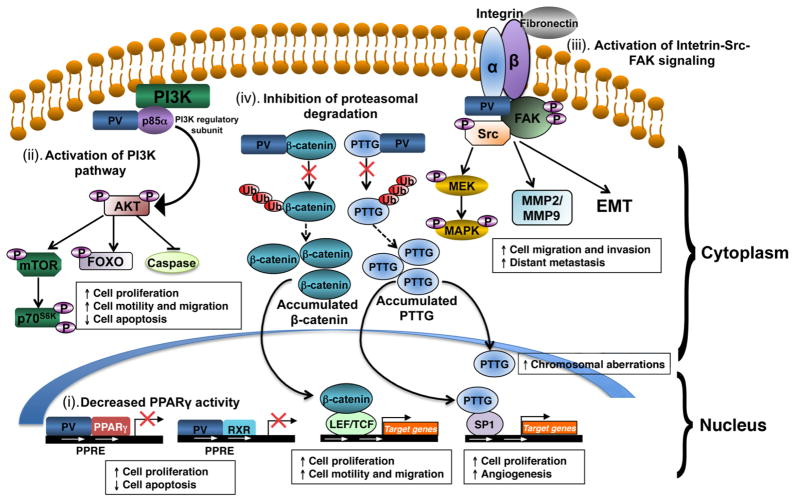
Figure 3. PV mediates oncogenic actions in thyroid carcinogenesis of ThrbPV/PV mice via multiple mechanisms.
(i). PV decreases peroxisome proliferator–activated receptor γ (PPARγ) activity by competition with PPARγ for binding to PPARγ response elements (PPRE) as heterodimers with PPARγ or the retinoid X receptor (RXR). Repressed transcriptional activity of PPARγ leads to increase cancer cell proliferation and decrease apoptosis in thyroids of ThrbPV/PV mice. (ii) PV activates phosphatidylinositol 3-kinase (PI3K) signaling pathway by physical interaction with the regulatory subunit of PI3K (p85α). Over-activation of PI3K-Akt signaling increases proliferation and migration of cancer cells and decreases apoptosis via the downstream pathways as shown. (iii) PV activates integrin-Src-focal adhesion kinase (FAK) signaling cascade. PV interacts with integrin and activates phosphorylation of Src and FAK. Activated Src-FAK signaling pathway induces epithelial-mesenchymal transition (EMT) and activation of matrix metalloproteases (MMPs) to increase cell migration, invasion and distant metastasis. Activated Src also potentiates mitogen-activated protein kinase (MAPK) signaling to increase cell proliferation and EMT. (iv) PV inhibits proteasomal degradation of the pituitary tumor transforming gene (PTTG) and β-catenin. PV binds to PTTG and prevents its degradation by proteasome, resulting in accumulation of PTTG. Elevated PTTG impedes mitotic progression, leading to chromosomal aberrations. Elevated PTTG, via transcription activation of its target genes, increases cell proliferation and augments angiogenesis. PV also increases the stability of β-catenin via direct protein-protein interaction, thereby inhibiting protesomal degradation of β-catenin. Elevated β-catenin protein levels increase transcription activation of its downstream target genes that affect cell proliferation and migration.