Abstract
BACKGROUND
Chronic constriction injury of trigeminal infraorbital nerve results in transient analgesia followed by whisker pad mechanical allodynia in rats. Neuregulin1 expressed on axonal membranes binds receptor tyrosine kinase ErbB promoting Schwann cell development and remyelination. This study investigated whether orofacial mechanical allodynia is signaled by ErbB3/ErbB2 heterodimers in injured nerves.
METHODS
Whisker pad mechanical allodynia (von Frey stimuli) was quantified in wild type rats and in transgenic rats with Sleeping Beauty transposon mutation for neuregulin1 transgene. Pain-related behavior was retested after intraperitoneal injection of ErbB2 inhibitor, Lapatinib, an agent shown by others to reduce breast cancer pain. Infraorbital nerve injury was evaluated histologically with myelin and neuronal biomarkers. ErbB3 changes over time were measured with western blots.
RESULTS
Whisker pad mechanical hypersensitivity began in week 2 in wild type rats (3.11 ± 5.93 vs. 18.72 ± 0.00g after sham surgery, n = 9, p < 0.001) indicating trigeminal neuropathic pain, but was not evident in transgenic rats (odds ratio: 1.12, 95% confidence interval: 0.38-3.35). Initiation of statistically significant mechano-hypersensitivity was delayed until week 6 after surgery in transgenic rats (3.44 ± 4.60g vs. 18.72 ± 0.00g, n = 4, p < 0.001). Mechanical allodynia which persisted 8 weeks in wild type rats was alleviated by Lapatinib (15 ± 3.89g vs. 2.45 ± 1.13g, n = 6, p < 0.001). Infraorbital nerve damage was verified histologically. Statistically significant ErbB3 increases (weeks 5 and 10) in wild type and transgenic rats (week 10) coincided with time points when mechanical hypersensitivity was present.
CONCLUSION
Neuregulin1/ErbB3/ErbB2 is a causal mechanism in nerve injury induced trigeminal neuropathic pain. Understanding peripheral glial mechanisms after nerve injury will improve neuropathic pain treatment.
Introduction
Trigeminal neuropathic pain is one of three main neuropathic pain-related diagnoses. The orofacial pain condition is characterized by chronic aching and burning pain as well as sudden excruciating, electric-like shooting pain caused by unintentional injury to the trigeminal system. There are few investigations on nociception mechanisms and analgesic trials in the trigeminal region. Thus, new analgesic strategies need to be explored before chronic trigeminal neuropathic pain can be successfully relieved.
Deterioration of trigeminal nerve myelin sheaths is one causal mechanism for trigeminal neuropathic pain. Mechanical hypersensitivity accompanying trigeminal neuropathic pain involves spontaneous and low-threshold activity in injured myelinated fibers 1. These pathological changes cause ectopic discharge or impulse generation from sites along axons where damage has occurred, rather than just at sensory nerve endings 2. Sciatic nerve constrictive injury induces demyelination and is a source of pathological ectopic firing leading to mechanical allodynia and heat hyperalgesia 3. Neuregulin 1 (Nrg1) is a peptide ligand signaling via the receptor tyrosine kinase ErbB3/ErbB2 heterodimer 4. The Nrg1/ErbB3/ErbB2 signaling complex plays a key role in axon-Schwann cell interactions promoting Schwann cell development and re-myelination after nerve injury. Nrg1 binds catalytically inactive ErbB3 while ErbB2 contributes tyrosine kinase activity in Schwann cells 5,6. Blockade of ErbB receptor-initiated cell signaling in glial cells wrapping either myelinated or non-myelinated nerves produces unique sensory dysfunctions 7. Thermal and mechanical hypersensitivity as well as thin myelination of sciatic nerves are found in ErbB receptor mutant mice 8,9. Upregulation of ErbB3 messenger RNA is maintained in different types of median nerve regenerating axons after upper limb injury while ErbB2 messenger RNA is unchanged 10. Possible changes of ErbB after nerve injury or a role in generating trigeminal neuropathic pain have not been explored and are the focus of this study. The present experiments utilized Nrg1 transgenic rats (Nrg1Tg) to explore the behavioral changes in whisker pad and ErbB receptor levels in injured nerve after infraorbital nerve chronic construction injury (CCI-ION). Lapatinib (Tykerb), an ErbB2 inhibitor, was applied after CCI-ION to investigate its effect on the pain related behavior. Lapatinib is used in ErbB2-positive breast cancer treatment and reportedly has reduced pain in a Phase II clinical trial 11. We further investigated the relationship of ErbB3 levels in injured nerves to the development of whisker pad mechanical allodynia following CCI-ION. We hypothesized that ErbB receptor level increase at infraorbital nerve injury sites promotes mechanical hypersensitivity in the whisker pad.
Materials and Methods
Animals
Adequate measures were taken to minimize pain or discomfort in these studies. Experiments were carried out in accordance with the Guidelines of the National Institute of Health regarding the care and use of animals for experimental procedures. Experiments were approved by the Institutional Animal Care and Use Committee at the University of Kentucky, Lexington, Kentucky. Male Fisher 344 wild type or Nrg1Tg rats were accommodated in ventilated animal housing with a reverse 12/12 light/dark cycle and weighed 200-250g at the beginning of the experiments. A breeding pair of Fisher 344 Nrg1Tg rats generated by introduction of a Sleeping Beauty transposon with a bidirectional splice acceptor gene trap12,13 was provided by the Knock Out Rat Consortium. A nonsense mutational insertion into intron 1 of the sequence encoding for Nrg1 domain resulted in ablation of all Nrg1 isoforms. The Nrg1Tg rats used in this study were bred in our animal facility, and the Nrg1Tn(sb-T2/Bart3)2.183Mcwi deficient genotype was confirmed.
Genotyping
Rats were genotyped by polymerase chain reaction using the following primers: 5’-AGGAACCAAAAAAGTAGACTCAGTGTG-3’, 5’-GTGAGCTTTTCTGTAAGGTGGTAACTT-3’, and transposon primer 5’-CTGACCTAAGACAGGGAATT-3’. Polymerase chain reaction was carried out at 94°C for 2 min, and then 94°C for 15 s, 60°C for 30 s, and 72°C for 90 s for 35 cycles, followed by 5 min extension at 72°C by PTC-100 programmable thermal controller (MJ Research Inc., Waltham, MA). The expected 1,041 bp product for wild-type allele, 1,041 and 440 bp products for heterozygous allele, and 440 bp product for the mutant allele were separated on a 1% agarose gel.
Surgery
Rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneal), and all surgeries were performed in sterile conditions under a surgical microscope. The ION on one side was exposed using the surgical procedure described by Vos 14. The ION was dissected free at its most rostral extent in the orbital cavity and two chromic gut (5-0) ligatures were loosely tied around the ION (2 mm apart). Nerve exposure but no ligation was performed in the sham operation. To obtain the desired degree of constriction a criterion formulated by Bennett and Xie 15 was applied, i.e., ligations reduced the nerve diameter just noticeably. The ligation retarded but did not totally occlude circulation through the superficial vasculature.
Assessment of Mechanical Allodynia of Rat Whisker Pad and Drug Administration
Mechanical sensitivity of vibrissal whisker pad, which is the ION receptive field, was measured with eight von Frey fibers (0.4, 0.6, 1, 2, 4, 6, 8, 15 g; Stoelting, Wood Dale, IL) by modified up-and-down method with a default maximal threshold at 18.72g 16. Mechanical stimuli were applied within the ION territory, near the whisker pad centers, both ipsilateral and contralateral to the surgery site. Responses to von Frey filaments applied to the rat whisker pad determined the threshold required for 50% head withdrawals. Each filament was applied five times at intervals of a few seconds. If head withdrawal was observed at least three times after probing with a filament, the rat was considered responsive to that filament. Whenever a positive response to a stimulus occurred, the next smaller von Frey filament was applied. Otherwise, the next higher filament was applied. Behavioral changes to mechanical stimuli were tested once a week for 10 weeks after surgery. Intraperitoneal injection of tyrosine kinase ErbB2 inhibitor Lapatinib (0, 0.2, 1, 5 mg/kg, in 300 μl dimethyl sulfoxide, Selleckchem, Houston, TX) 17 was administered to CCI-ION rats after mechanical allodynia was confirmed. Behavioral responses were determined for whisker pads on both sides at 30 min, 1 h, 3 h, and 6 h after Lapatinib administration.
Morphological Analysis
Aldehyde Fixation. Rats were anesthetized with sodium pentobarbital (70 mg/kg, intraperitoneal) and perfused transcardially with heparinized saline followed by 4% ice-cold paraformaldehyde in 0.1 M phosphate buffer solution (pH 7.4). Paraffin Embedding. Infraorbital nerves were dissected out and placed in the same fixative solution at 4°C overnight. Samples were switched to 70% ethanol, dehydrated through graded ethanol, and embedded in paraffin. Infraorbital nerve tissue sections were cut (5 μm), mounted onto glass slides (Superfrost Plus, VWR, Radnor, PA), deparaffinized (Citrisolv, Fisher Scientific, Pittsburg, PA), dehydrated with graded ethanol, and rinsed in ddH2O. Hematoxylin and Eosin Staining. Slides were immersed in 0.1% hematoxylin, washed in tap water, immersed in 0.1% eosin and washed in distilled water. Sections were dehydrated in ethanol and coverslipped (Permount, Fisher Scientific). Immunofluorescence. After block of nonspecific antigen sites with 3% normal goat serum (30 min), sections were incubated overnight (4°C) with rabbit anti-myelin protein zero (1:1,000, Abcam, Cambridge, MA) and mouse anti-pan-axonal-neurofilament protein (1:1,000, Convance, Princeton, NJ) antibodies. Subsequently, sections were incubated with secondary antibodies, fluorescein isothiocyanate donkey anti-mouse antibody and Texas Red donkey anti-rabbit antibody (1:1,000, 1 h, Santa Cruz, Santa Cruz, CA). Sections were coverslipped with glycerol based mounting media (Vector Laboratories, Burlingame, CA). Staining was visualized using a Nikon E1000 microscope (Nikon Instruments, Inc., Melville, NY) equipped with MetaVue and Act-1 Programs. Photomicrographs were taken under the same conditions. Fluorescent intensity was analyzed offline using an advanced image-analysis system MetaMorph (Molecular Devices, Sunnyvale, CA). The numbers of axons with intact myelin sheaths in the best five representative sections of three rats from each group were counted 18,19. The person who counted the number of myelinated axons was blinded to experimental groups.
Western blot
Infraorbital nerve fragments from the ligation site were dissected and homogenized in radioimmunoprecipitation assay buffer. Total protein samples (50 μg) were loaded onto SDS-PAGE (Bio-Rad, Hercules, CA), transferred to polyvinylidene fluoride membrane, blocked overnight and probed with rabbit anti-ErbB3 (1:200) and mouse anti-β-actin (1:10,000, Santa Cruz, Santa Cruz, CA) (2 h). The membrane was incubated with anti-rabbit and anti-mouse peroxidase-conjugated secondary antibody (1:10,000; GE Healthcare, Piscataway, NJ) (1 h). Immunoreactive proteins were detected by enhanced chemiluminescence kit (Amersham Biosciences, Pittsburgh, PA). Bands recognized by the primary antibody were visualized by X-ray film exposure and analyzed with Image J (National Institutes of Health, Baltimore, MD). The intensities of the ErbB3 bands were normalized to β-actin intensities and the relative intensity of ErbB3 from each group were compared.
Statistics
All data were expressed as mean ± SD, analyzed using the Prism 4 statistical program (Graph Pad Software, Inc., La Jolla, CA). The behavioral changes after nerve injury among four groups (Nrg1Tg and wild type rats with/without nerve ligation) for ipsilateral or contralateral sides were analyzed on a weekly basis by two-way ANOVA followed by Bonferroni post-tests. The odds ratio for the effect in week 2 for wild type versus transgenic rats was calculated using McNemar's test with a 95% confidence interval. The mechanical threshold changes on both sides of four groups (Nrg1Tg and wild type rats with/without nerve ligation) over 10 weeks and the time-course data from 0.5, 1, 3, and 6 h after Lapatinib administration were analyzed by repeated-measures one-way ANOVA followed by Tukey's Multiple Comparison post hoc tests. For the dose-dependent responses and the western blot data from each group and groups compared to initial level, comparisons were executed by independent t-test with two-tailed p values. The same comparisons were done for the contralateral side. A p ≤ 0.05 was considered significant. There were no missing data from any of the experimental results.
Results
Orofacial Hypersensitivity after CCI-ION in Wild Type Rats
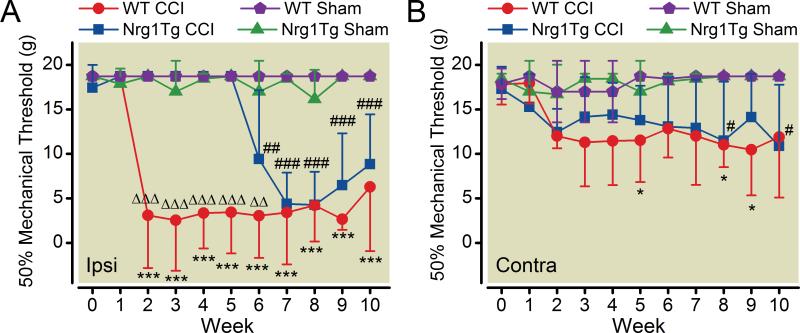
Behavioral testing to determine mechanical threshold of the whisker pad to von Frey fiber stimuli was performed once a week for 10 weeks after CCI-ION in both Nrg1Tg and wild type rats. Behavioral alterations observed after nerve injury were indicative of severe sensory disturbances within the injured nerve receptive territory (i.e., whisker pad). After constrictive nerve injury, rats exhibited responses to mechanical stimuli in two phases. 1) First phase: During the first 2 weeks after CCI-ION there is no change in responses to mechanical stimuli applied to whisker pad with any of the eight test filaments. 2) Second phase (mechanical allodynia): Two weeks or more after CCI-ION surgery, low intensity stimuli applied to the injured nerve territory evokes a sensitized response in wild type rats. Wild type rats showed mechanical allodynia (3.11 ± 5.93g, n = 9) in week 2 after nerve injury that persisted at least 8 weeks, i.e., a statistically significant decrease in mechanical threshold compared to responses of the sham group (18.72 ± 0.00g, n = 4) (p < 0.001) (fig. 1A).
Fig. 1.
(A) Timecourse for development of mechanical allodynia. Mechanical allodynia develops with different time courses in the vibrassal pads of wild type (WT) and neuregulin1 transgenic (Nrg1Tg) rats after chronic constriction injury of infraorbital nerve (CCI-ION). Mechanical sensation threshold on the vibrassal pad in response to von Frey fiber stimulation was tested in both types of rats after CCI-ION. Mechanical allodynia was evident on the ipsilateral side of the nerve injury. Rats exhibit behavioral changes developing in two phases: 1) First hypo-responsive phase: After nerve injury, initially there was no change from baseline recorded in response to mechanical stimuli applied to the whisker pad with any strength test filament. The first phase persisted 5 weeks in Nrg1Tg rats versus 1 week in wild type rats. 2) Second hyper-responsive phase: Mechanical thresholds were markedly decreased during this phase for all groups except the surgical shams. Mechanical allodynia was present by week 2 in wild type rats. Development of allodynic responses was delayed until week 7 in Nrg1Tg rats. There was no difference between wild type and Nrg1Tg rats in weeks 7-10 of the hyper-responsive phase. p<0.001 vs. sham group of wild type rats; ##p<0.01, ###p<0.001 in week 7-10 vs. sham group of Nrg1Tg rats; ΔΔp<0.01, ΔΔΔp<0.001 vs. CCI group of Nrg1Tg rats. (mean±S.D)
(B) Contralateral whisker pad hypersensitivity after surgery. Increased responses (lowered mechanical threshold) were noted on the contralateral whisker pads after CCI in both wild type and Nrg1Tg rats. *p<0.05 vs. sham group in wild type rats; #p<0.05 vs. sham group in Nrg1Tg rats. (mean±S.D)
Delayed Hypersensitivity after CCI-ION in Nrg1Tg Rats
There was no mechanical threshold difference between wild type and Nrg1Tg rats prior to surgery or week 1 after CCI-ION. In Nrg1Tg rats development of second phase hypersensitive responses (4.39 ± 3.51g, n = 8) (lowered threshold) occurred with a statistically significant delay (week 6) compared to wild type rats, i.e., the first phase lasted for 5 weeks rather than 1 week. Thus, differences in mechanical thresholds on the whisker pads were statistically significant between wild type and Nrg1Tg rats in week 2-5 (3.44 ± 4.60g vs. 18.72 ± 0.00g, p < 0.001) and in week 6 (3.05 ± 4.73g vs. 9.42 ± 7.74g, p < 0.01) postsurgery. Second phase hypersensitivity to mechanical stimuli persisted for 5 weeks in Nrg1Tg rats, from week 6 to week 10 (p < 0.01 in week 6 and p < 0.001 in week 7-10 compared to sham group of Nrg1Tg rats, n = 4). Mechanical thresholds on the contralateral whisker pads were decreased compared to thresholds of the contralateral sides for both sham groups (wild type and Nrg1Tg rats) (11.54 ± 4.70g, 11.01 ± 2.49g, 10.46 ± 5.10g, p < 0.05 in week 5, 8, 9 in wild type rats and 11.47 ± 6.65g, 10.89 ± 6.87g, p < 0.05 in week 8, 10 in Nrg1Tg rats) (fig. 1B).
Reversal of Mechanical Allodynia by Functional ErbB3/ErbB2 Heterodimer Inhibitor
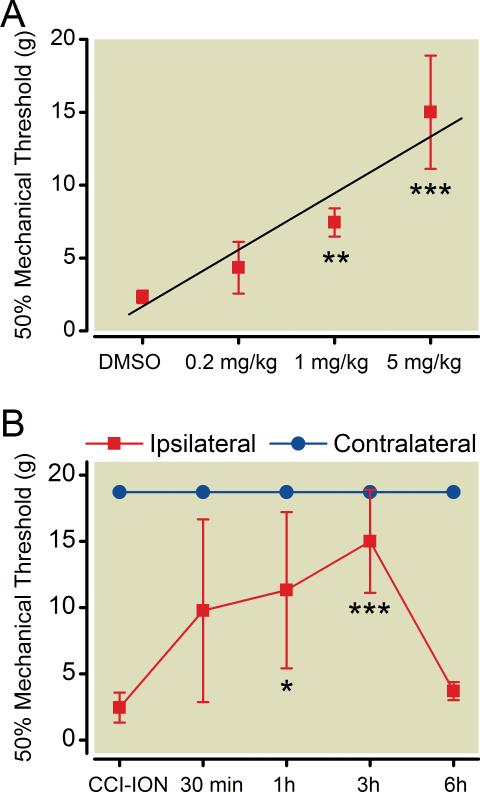
Effect of ErbB2 inhibitor Lapatinib on mechanical allodynia was tested at different doses comparing 0.2, 1 and 5 mg/kg to vehicle treatment. Lapatinib was injected intraperitoneally to wild type rats with CCI-ION in week 4 postsurgery. Mechanical allodynia was confirmed before drug administration. Mechanical thresholds to von Frey filaments were tested on whisker pads at 30 min, 1 h, 3 h, and 6 h after drug injection. Lapatinib dose-dependently alleviated mechanical allodynia in wild type rats with CCI-ION at 3 h after drug administration (fig. 2A). Mechanical threshold increased ipsilaterally at 30 min, 1 h, and 3 h in response to 5 mg/kg Lapatinib. The effect reached a peak at 3 h (15 ± 3.89g vs. 2.45 ± 1.13g, p < 0.001, n = 6) and diminished at 6 h (fig. 2B). There was no difference in mechanical threshold on the contralateral sides either before or after Lapatinib administration (dimethyl sulfoxide vehicle, 0.2, 1, and 5 mg/kg).
Fig. 2. ErbB2 tyrosine kinase inhibitor reduces mechanical allodynia.
Whisker pad mechanical allodynia is alleviated by an active ErbB2 tyrosine kinase inhibitor, Lapatinib, in wild type (WT) rats with chronic constriction injury of infraorbital nerve (CCI-ION). In week 4 post CCI-ION, mechanical threshold in wild type rats was tested to confirm mechanical allodynia to von Frey filaments. Lapatinib was administered by intraperitoneal injection (vehicle, 0.2, 1 and 5mg/kg) after the pre-drug threshold was determined on both whisker pads. Bilateral behavioral responses to mechanical stimuli were monitored 30min, 1h, 3h and 6h post Lapatinib administration. (A) Lapatinib dose-dependently (Dimethyl sulfoxide (DMSO), 0.2, 1 and 5mg/kg) decreased mechanical allodynia in wild type rats with CCI-ION at 3h after drug administration. **p<0.01 at 1mg/kg, ***p<0.001 at 5mg/kg vs. DMSO. (mean±S.D) (B) Mechanical thresholds were increased by Lapatinib administration on the nerve injured side at 30min, 1h and 3h, with a maximal effect found at 3h post. *p<0.05 at 1h, ***p<0.001 at 3h vs. pre-drug. There was no change in mechanical threshold on the contralateral side after intraperitoneal injection of Lapatinib. (mean±S.D)
Infraorbital nerve injury by loose ligation
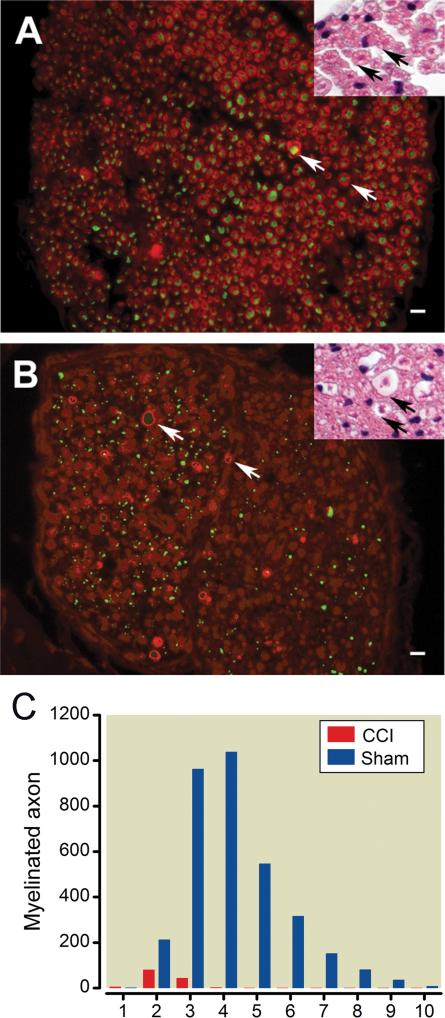
Hematoxylin and eosin staining was performed on cross sections obtained from infraorbital nerve injury sites and equivalent nerve segments from the sham group 5 weeks after surgery. Sections from the sham group showed the normal peripheral nerve structure with dense labeling of axons and paler staining of myelin sheaths (fig. 3A inset). In injured nerves, axons were swollen, myelin was diminished, and normal structure was disrupted (fig. 3B inset). However, myelin disruption was more evident with immunofluorescence identifying myelin protein zero (red, fig. 3A). CCI-ION damaged myelin sheaths were evident when stained for myelin protein zero and counter-staining of axons with pan-axonal-neurofilament antibody (green) (fig. 3B). Most myelinated axons were damaged in the injured nerves and few remained with intact myelin sheaths (fig. 3C, table 1). Large myelinated axons (5-10 μM) were absent.
Fig. 3. Morphological alterations produced by chronic constriction injury.
(A) Morphology of the sham control infraorbital nerve (ION): The cytoarchitecture of the ION in the surgical sham control group is shown in nerve cross sections. Axons stained for neurofilament 200 by immunofluorescence (green) are surrounded by myelin sheaths (white arrow) stained for myelin protein zero (red). Inset: Hematoxylin and eosin (H&E) staining shows the normal structure of a fascicle of the infraorbital nerve fiber. Each myelinated nerve fiber axon is densely stained and encircled by the lighter Schwann cell myelin sheath ring (black arrows). (B) Morphology after ION chronic constriction injury (CCI): Most MPZ stained myelin sheaths have lost their circular morphology and axonal staining in the center of their structure, i.e. the immunopositive structures (red) wrapping around the axons (green) have collapsed. Axons are seen scattered in the endoneurium without myelin sheaths or with a few wraps of a damaged myelin sheath (white arrows). Inset: Myelin sheath in infraorbital nerve disrupted by ligation shown 5 weeks after surgery (black arrows). Bars: 10 μm (C) ION size distribution: The histogram shows the infraorbital nerve myelinated axon fiber size distribution and changes after constriction injury.
Table 1.
Myelinated Axon Distribution in Infraorobital Nerve after Nerve Injury
| Axon Diameter (μm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Myelinated Axons | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Sham | 1 | 212 | 963 | 1038 | 546 | 316 | 152 | 81 | 36 | 8 |
| CCI-ION | 5 | 80 | 43 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
CCI-ION = chronic constriction injury of infraorbital nerve
Infraorbital Nerve ErbB3 Increases in Rats with CCI-ION
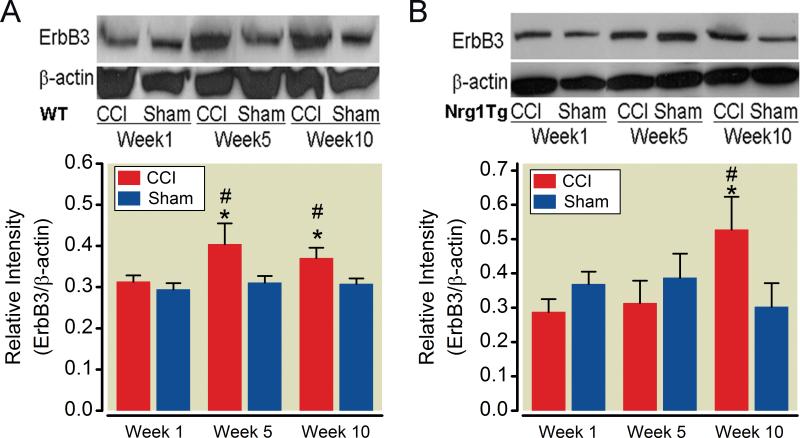
The ErbB3 receptor protein levels at the CCI-ION site were examined using western blots normalized to β-actin. The ErbB3 receptor levels in injured infraorbital nerves from wild type (in week 1) and Nrg1Tg rats (in week 1 and week 5) were similar to rats with sham surgery. ErbB3 level increases were statistically significant in weeks 5 and 10 relative to sham wild type rats and rats with CCI-ION in week 1 (p = 0.038 in week 5 and p = 0.013 in week 10 respectively, p = 0.041, 0.014 vs. CCI group in week 1, n = 3) (fig. 4A, B). ErbB3 level increases were statistically significant in week 10 (p = 0.03 vs. sham group in week 10, the CCI group in week 1 p = 0.034 and 5 p = 0.016, n = 3) in Nrg1Tg rats but not in week 5 (p = 0.26 vs. sham group in week 5, p = 0.59 vs. the CCI group in week 1) (fig. 4C, D). Statistically significant increases in ErbB3 receptor levels were concurrent with the behavioral hypersensitivity, i.e., ErbB3 was increased in week 2 after injury in wild type rats and in week 5 in Nrg1Tg rats. These differences were not observed in the contralateral nerves (fig. 5).
Fig. 4. Infraorbital nerve ErbB3 receptor level increases after nerve injury are delayed in neuregulin1 transgenic (Nrg1Tg) rats.
Infraorbital nerve ErbB3 receptor level was determined by western blot analysis at different time points relevant to behavioral changes in rats with both phenotypes. (A) ErbB3 in wild type rats. ErbB3 receptor levels are shown for wild type (WT) animals with chronic constriction injury (CCI) and the surgical sham groups. (B) For wild type rats, there was no difference in infraorbital nerve ErbB3 receptor expression for the sham and surgery groups in week 1. Statistically significant increases in ErbB3 receptors were observed in week 5 and week 10 after injury in wild type rats with CCI compared to the sham group. (C) ErbB3 in Nrg1Tg rats. ErbB3 receptor levels in Nrg1Tg rats are shown for the CCI and the surgical sham groups. (D) The increase in ErbB3 receptor level in Nrg1Tg rats was not statistically significant compared to the sham group until week 10 after ligation. The increases in infraorbital nerve ErbB3 receptor levels were concurrent with the mechanical threshold changes in response to von Frey fiber stimuli. *p<0.05 vs. CCI group on week 1 in wild type and Nrg1 Tg rats. #p<0.05 vs. sham group in both week 5 and 10 in wild type rats, and week 10 in Nrg1 Tg rats. (mean±S.D)
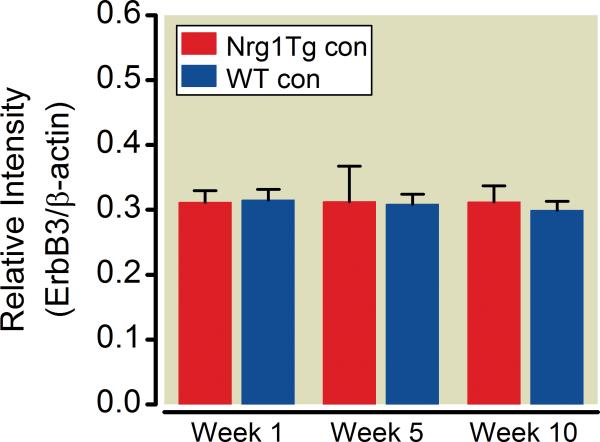
Fig. 5. No contralateral changes in ErbB3 receptor levels.
No contralateral (CON) changes were evident in infraorbital nerve ErbB3 receptor levels in the wild type (WT) or neuregulin1 transgenic (Nrg1Tg) rats. Infraorbital nerve ErbB3 receptor level on the contralateral side was determined by western blot analysis at different time points relevant to the behavioral changes in rats with both phenotypes. There was no difference in infraorbital nerve ErbB3 receptor expression comparing wild type (WT) and Nrg1Tg rats in week 1, 5 and 10 after nerve injury on the contralateral side. (mean±S.D)
Discussion
The present study characterizes behavioral, structural, and neurochemical changes after CCI-ION in both wild type and Nrg1Tg rats. Mechanical allodynia persisting at least 8 weeks in wild type rats in our study is consistent with the 120 day CCI-ION model time course reported by Vos 14. Myelin sheaths were heavily damaged or destroyed by ligation in both wild type and Nrg1Tg rats. Trigeminal root biopsies from neuropathic pain patients exhibit pathological changes such as axonal loss and demyelination 20,21. Constrictive sciatic nerve injury induces demyelination as sources of pathological ectopic firing accompanying mechanical allodynia and heat hyperalgesia 3. Nrg1 is involved in peripheral Schwann cell/ axon communication, growth, migration, and myelination 22. Mice deficient in Bace1 which cleaves the extracellular domain of Nrg1 show myelin impairment and nociceptive hypersensitivity 23. The present study provided data in support of a role for the Nrg1/ErbB3/ErbB2 signaling complex in axon-Schwann cell interactions promoting mechanical allodynia after nerve injury.
There was initially no difference in infraorbital nerve ErbB3 expression from baseline in Nrg1Tg and wild type CCI-ION rats during the first phase. While mechanical allodynia develops immediately after CCI injury in sciatic nerve 24, infraorbital nerve constrictive injury induced mechanical hypersensitivity is typically evident several days later 14. Although the first phase is speculated to be a residual effect of anesthetics or increases of endogenous opioids 25, differences in the regions affected (orofacial vs. somatic) and the mechanisms involved might be relevant. The increases in mechanical allodynia and ErbB3 were both statistically significant in week 5 after infraorbital nerve injury in wild type rats. However, development of mechanical allodynia was delayed in Nrg1Tg rats after CCI-ION. Our results showed that Nrg1Tg rats have a prolonged first phase during which there is no ErbB3 expression increase and no mechanical allodynia. Thus, increases in ErbB3 receptor levels after nerve injury were concurrent with whisker mechanical allodynia suggesting ErbB3 receptor activation is integrally involved in mechanical sensitization. Increases in ErbB3 phosphorylation and expression levels have been reported in distal nerves after transection of sciatic nerves 26,27.
ErbB3 activation is a crucial time-dependent event among nerve injury signaling in wild type and Nrg1Tg rats suggesting Nrg1 involvement in pain-related behavioral changes following injury. Nrg1 has been shown to increase ErbB3 and olfactory glial proliferation in culture 28. Nrg1 delivered by virus in transected nerves promoted axonal and Schwann cell growth and increased responses to nociceptive stimuli after sciatic nerve axotomy 29. Low Nrg1 in Nrg1Tg rats likely provides minimal ErbB3 activation after nerve injury. It is still unclear what compensatory mechanisms promote increased ErbB3 and sensitized behavior in week 10 in Ngr1Tg rats. It is possible that the Nrg1/ErbB response is slowed even more in the mutant Nrg1Tg rats during recovery from nerve trauma.
ErbB3 signals through its active tyrosine kinase heterodimer ErbB2 to trigger downstream cascades. However, in a study by ErbB2 mutant mice with sciatic nerve transection, ErbB2 was found to be nonessential for Schwann cell proliferation and survival 30. Other potential ErbB mechanisms that might be involved include nerve ligation-induced microglial proliferation and activation initiated by Nrg1-ErbB and ERK1/2/p38 mediation of downstream signaling pathways 31,32.
Lapatinib is used in ErbB2 (HER2)-positive breast cancer therapy and in a phase 2 clinical trial reportedly improved patient functioning, symptom relief, and pain 11. In the present study, the tyrosine kinase inhibitor, Lapatinib, provides significant alleviation of mechanical allodynia indicating ErbB3/2 involvement in whisker pad mechanical allodynia. Blocking ErbB3 downstream signaling with Lapatinib dose dependently ameliorated the behavioral allodynia in wild type rats. These results also suggest a relationship between whisker pad mechanical allodynia and infraorbital nerve injury-induced peripheral glial activation through ErbB3/ErbB2. The blunting of the behavioral pain-related behavior only on the ipsilateral side suggests a direct effect of the Lapatinib on the injured nerve firing and /or peripheral glial activation, while having no effect on ongoing central sensitization events at the time points tested. On the contralateral side which was uninjured, there was no difference in infraorbital nerve ErbB3 receptor expression comparing wild type and Nrg1Tg rats in week 1, 5, and 10 after the nerve injury. This finding serves as an internal control confirming that the ErbB3 increases in response to the injury.
One important factor contributing to the pathogenesis of trigeminal neuropathic pain is an abnormal compression of the trigeminal nerve by a vein or artery leading to deterioration of myelin sheaths 20. The investigation of peripheral glial ErbB3/ErbB2 mechanisms in the current study using the constrictive nerve injury model provides implications for potential clinical therapies to alleviate the leading cause of trigeminal neuropathic pain. Reduction of the behavioral hypersensitivity by the ErbB2 inhibitor and the increase in ErbB3 protein after nerve injury suggest enhanced ErbB3/ErbB2 complex signaling following infraorbital nerve injury mediates mechanical allodynia in rat whisker pad.
Interventions addressing peripheral ErbB3/ErbB2 mechanisms could potentially be better choices as nonsurgical therapy instead of microvascular depression surgery, which is sometimes an effective treatment for trigeminal neuropathic pain. Similarly, other nerve constrictive injuries may also be alleviated by an ErbB2 inhibitor.
MS #201110014 Final box summary.
What we already know about this topic
Peripheral nerve injury results in demyelination and activation of Schwann and other support cells around peripheral nerves
The role of this process in neuropathic pain remains obscure
What this article tells us that is new
In rats, genetic disruption of neuregulin-1, which signals proliferation and activation of Schwann cells, delayed downstream receptor signaling and hypersensitivity to touch on the whisker pad after infraorbital nerve injury
Lapatinab, an experimental drug in Phase II trials the treatment of breast cancer and which targets this downstream receptor signaling pathway, reduced hypersensitivity in rats with nerve injury
Acknowledgments
These studies were funded by National Institutes of Health, National Center for Research Resources 2P20RR020145, the University of Kentucky President's Research Fund (KNW) and start-up funds from the Dean of the College of Medicine (KNW), University of Kentucky, Lexington, Kentucky.
The authors express thanks for the technical assistance from Ryan Nesemeier, B.S., Technician, Department of Physiology, University of Kentucky, Lexington, Kentucky and comments on the manuscript from Robert Danaher, Ph.D., Research Assistant Professor and Craig Miller, D.M.D., M.S., Professor (tenured), College of Dentistry, University of Kentucky, Lexington, Kentucky.
Footnotes
Preliminary results of these studies were presented at the 2011 Society for Neuroscience meeting in Chicago, Illinois, November 12, 2009.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakagawa K, Takeda M, Tsuboi Y, Kondo M, Kitagawa J, Matsumoto S, Kobayashi A, Sessle BJ, Shinoda M, Iwata K. Alteration of primary afferent activity following inferior alveolar nerve transection in rats. Mol Pain. 2010;6:9. doi: 10.1186/1744-8069-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain. 2006;7:S3–S12. doi: 10.1016/j.jpain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Costa B, Trovato AE, Colleoni M, Giagnoni G, Zarini E, Croci T. Effect of the cannabinoid CB1 receptor antagonist, SR141716, on nociceptive response and nerve demyelination in rodents with chronic constriction injury of the sciatic nerve. Pain. 2005;116:52–61. doi: 10.1016/j.pain.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 4.Falls DL. Neuregulins: Functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 5.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 6.Garratt AN, Britsch S, Birchmeier C. Neuregulin, a factor with many functions in the life of a schwann cell. Bioessays. 2000;22:987–96. doi: 10.1002/1521-1878(200011)22:11<987::AID-BIES5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Campana WM. Schwann cells: Activated peripheral glia and their role in neuropathic pain. Brain Behav Immun. 2007;21:522–7. doi: 10.1016/j.bbi.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, Corfas G. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat Neurosci. 2003;6:1186–93. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- 9.Chen S, Velardez MO, Warot X, Yu ZX, Miller SJ, Cros D, Corfas G. Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. J Neurosci. 2006;26:3079–86. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Audisio C, Nicolino S, Scevola A, Tos P, Geuna S, Battiston B, Perroteau I. ErbB receptors modulation in different types of peripheral nerve regeneration. Neuroreport. 2008;19:1605–9. doi: 10.1097/WNR.0b013e32831313ef. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman B, Wu Y, Amonkar MM, Sherrill B, Bachelot T, Salazar V, Viens P, Johnston S. Impact of lapatinib monotherapy on QOL and pain symptoms in patients with HER2+ relapsed or refractory inflammatory breast cancer. Curr Med Res Opin. 2010;26:1065–73. doi: 10.1185/03007991003680323. [DOI] [PubMed] [Google Scholar]
- 12.Taylor SB, Taylor AR, Markham JA, Geurts AM, Kanaskie BZ, Koenig JI. Disruption of the neuregulin 1 gene in the rat alters HPA axis activity and behavioral responses to environmental stimuli. Physiol Behav. 2010;104:205–14. doi: 10.1016/j.physbeh.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu B, Geurts AM, Poirier C, Petit DC, Harrison W, Overbeek PA, Bishop CE. Generation of rat mutants using a coat color-tagged Sleeping Beauty transposon system. Mamm Genome. 2007;18:338–46. doi: 10.1007/s00335-007-9025-5. [DOI] [PubMed] [Google Scholar]
- 14.Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat's infraorbital nerve. J Neurosci. 1994;14:2708–23. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 16.Gao X, Kim HK, Chung JM, Chung K. Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain. 2005;116:62–72. doi: 10.1016/j.pain.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 17.Dahal BK, Cornitescu T, Tretyn A, Pullamsetti SS, Kosanovic D, Dumitrascu R, Ghofrani HA, Weissmann N, Voswinckel R, Banat GA, Seeger W, Grimminger F, Schermuly RT. Role of epidermal growth factor inhibition in experimental pulmonary hypertension. Am J Respir Crit Care Med. 2009;181:158–67. doi: 10.1164/rccm.200811-1682OC. [DOI] [PubMed] [Google Scholar]
- 18.Klein BG, Renehan WE, Jacquin MF, Rhoades RW. Anatomical consequences of neonatal infraorbital nerve transection upon the trigeminal ganglion and vibrissa follicle nerves in the adult rat. J Comp Neurol. 1988;268:469–88. doi: 10.1002/cne.902680402. [DOI] [PubMed] [Google Scholar]
- 19.Shi JY, Liu GS, Liu LF, Kuo SM, Ton CH, Wen ZH, Tee R, Chen CH, Huang HT, Chen CL, Chao D, Tai MH. Glial cell line-derived neurotrophic factor gene transfer exerts protective effect on axons in sciatic nerve following constriction-induced peripheral nerve injury. Hum Gene Ther. 2011;22:721–31. doi: 10.1089/hum.2010.036. [DOI] [PubMed] [Google Scholar]
- 20.Devor M, Govrin-Lippmann R, Rappaport ZH. Mechanism of trigeminal neuralgia: an ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. J Neurosurg. 2002;96:532–43. doi: 10.3171/jns.2002.96.3.0532. [DOI] [PubMed] [Google Scholar]
- 21.Love S, Coakham HB. Trigeminal neuralgia: Pathology and pathogenesis. Brain. 2001;124:2347–60. doi: 10.1093/brain/124.12.2347. [DOI] [PubMed] [Google Scholar]
- 22.Birchmeier C. ErbB receptors and the development of the nervous system. Exp Cell Res. 2009;315:611–8. doi: 10.1016/j.yexcr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 23.Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–5. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 24.Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Kim KW, Han HJ, Beitz AJ, Na HS, Lee JH. Intrathecal injection of the sigma(1) receptor antagonist BD1047 blocks both mechanical allodynia and increases in spinal NR1 expression during the induction phase of rodent neuropathic pain. Anesthesiology. 2008;109:879–89. doi: 10.1097/ALN.0b013e3181895a83. [DOI] [PubMed] [Google Scholar]
- 25.Kernisant M, Gear RW, Jasmin L, Vit JP, Ohara PT. Chronic constriction injury of the infraorbital nerve in the rat using modified syringe needle. J Neurosci Methods. 2008;172:43–7. doi: 10.1016/j.jneumeth.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carroll SL, Miller ML, Frohnert PW, Kim SS, Corbett JA. Expression of neuregulins and their putative receptors, ErbB2 and ErbB3, is induced during Wallerian degeneration. J Neurosci. 1997;17:1642–59. doi: 10.1523/JNEUROSCI.17-05-01642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guertin AD, Zhang DP, Mak KS, Alberta JA, Kim HA. Microanatomy of axon/glial signaling during Wallerian degeneration. J Neurosci. 2005;25:3478–87. doi: 10.1523/JNEUROSCI.3766-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Mello TR, Busfield S, Dunlop SA, Plant GW. Culture conditions affect proliferative responsiveness of olfactory ensheathing glia to neuregulins. Glia. 2007;55:734–45. doi: 10.1002/glia.20502. [DOI] [PubMed] [Google Scholar]
- 29.Joung I, Yoo M, Woo JH, Chang CY, Heo H, Kwon YK. Secretion of EGF-like domain of heregulinbeta promotes axonal growth and functional recovery of injured sciatic nerve. Mol Cells. 2010;30:477–84. doi: 10.1007/s10059-010-0137-5. [DOI] [PubMed] [Google Scholar]
- 30.Atanasoski S, Scherer SS, Sirkowski E, Leone D, Garratt AN, Birchmeier C, Suter U. ErbB2 signaling in Schwann cells is mostly dispensable for maintenance of myelinated peripheral nerves and proliferation of adult Schwann cells after injury. J Neurosci. 2006;26:2124–31. doi: 10.1523/JNEUROSCI.4594-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calvo M, Zhu N, Grist J, Ma Z, Loeb JA, Bennett DL. Following nerve injury neuregulin-1 drives microglial proliferation and neuropathic pain via the MEK/ERK pathway. Glia. 2011;59:554–68. doi: 10.1002/glia.21124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calvo M, Zhu N, Tsantoulas C, Ma Z, Grist J, Loeb JA, Bennett DL. Neuregulin-ErbB signaling promotes microglial proliferation and chemotaxis contributing to microgliosis and pain after peripheral nerve injury. J Neurosci. 2010;30:5437–50. doi: 10.1523/JNEUROSCI.5169-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]