Fig. 1.
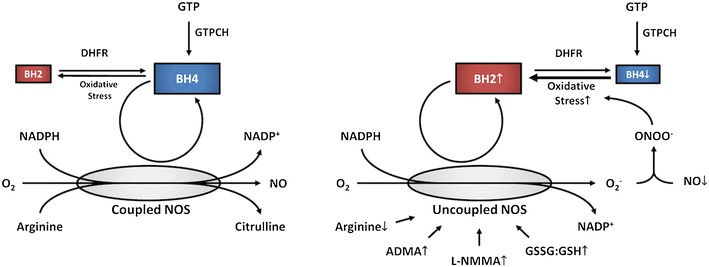
BH4 synthesis, recycling, and oxidation as determinants of NOS uncoupling. Left To produce nitric oxide (NO), nitric oxide synthase (NOS) enzymes require the substrates L-arginine and molecular oxygen (O2) and the cofactors tetrahydrobiopterin (BH4), reduced nicotinamide adenine diphosphate (NADPH), heme (not pictured), flavin mononucleotide (FMN, not pictured), and flavin adenine dinucleotide (FAD, not pictured). Under normal conditions, BH4 bioavailability is maintained by 1) de novo synthesis from guanosine triphosphate (GTP), in which the rate-limiting step is catalyzed by GTP cyclohydrolase (GTPCH) and 2) dihydrofolate reductase (DHFR)-mediated recycling of 7,8-dihydrobiopterin (BH2), the primary product of nonenzymatic BH4 oxidation. Right “Uncoupled” NOS is characterized by production of superoxide (O−2). NOS uncoupling is promoted by reduced BH4 bioavailability relative to either BH2 or NOS protein. In turn, O−2 produced by uncoupled NOS reacts with NO, forming peroxynitrite (ONOO−), a highly reactive anion that rapidly oxidizes BH4. Therefore, a state of NOS uncoupling is stabilized by self-propagating oxidative stress. In addition to this primary BH4-mediated cycle, additional mechanisms have been shown to promote uncoupling, including reduced arginine bioavailability, high levels of oxidized glutathione (GSSG) relative to reduced glutathione (GSH), or increased concentrations of the endogenous NOS inhibitors L-N-monomethylarginine (L-NMMA) and asymmetric dimethylarginine (ADMA)
