Abstract
Suppressed T cell functions in human immunodeficiency virus (HIV) infection were identified and corrected by lenalidomide in middle-aged HIV-infected patients. Chemotaxis of T cells from HIV-infected men (n = 6, mean 43 years) to sphingosine 1-phosphate (S1P) and CCL21 was significantly lower than that of HIV-negative men (n = 6, mean 41 years), and was enhanced significantly up to control levels by 100 and 1000 nM lenalidomide. Generation of interleukin (IL)-2, but not interferon (IFN)-γ, by T cells of middle-aged HIV-infected men was significantly lower than that for controls and was increased significantly by 10–1000 nM lenalidomide up to a maximum of more than 300%. CD4 and CD8 T cells isolated from healthy middle-aged men and reconstituted in vitro at a low CD4 : CD8 ratio typical of HIV infection had depressed chemotaxis to S1P, but not CCL21, and generation of IL-2, but not IFN-γ. Significant enhancement of chemotaxis to S1P and CCL21was induced by 100–1000 nM lenalidomide only for normal T cells at a low CD4 : CD8 ratio. T cells from HIV-negative middle-aged CD4 T lymphocytopenic patients (n = 3), with a CD4 : CD8 ratio as low as that of HIV-infected patients, had similarly diminished chemotaxis to S1P and CCL21, and depressed generation of IL-2, but not IFN-γ. Lenalidomide at 30–1000 nM significantly enhanced chemotaxis to S1P and IL-2 generation for T cells from HIV-negative CD4 T lymphocytopenic patients as from HIV-infected patients, with less effect on CCL21-elicited chemotaxis and none for IFN-γ generation. Defects in functions of T cells from middle-aged HIV-infected men are partially attributable to CD4 T lymphocytopenia and are corrected by lenalidomide.
Keywords: AIDS, chemotaxis, cytokines, immunopharmacology, T lymphocytes
Introduction
The extent and continuing severity of the worldwide epidemic of acquired immune deficiency syndrome (AIDS), despite widespread treatment, requires investigation of any potential therapeutic approach [1,2]. Most research in the treatment of HIV infection has focused appropriately on suppressing the causative virus and maintaining blood levels of CD4 T cells necessary for adaptive host defences. Several studies have revealed qualitative defects in immune functions of T cells from HIV-infected patients, some of which were detectable in selected patients with early human immunodeficiency virus (HIV) infection and normal CD4 T cell counts.
Initial analyses of T cell function in HIV infection involved principally homosexual male patients and focused on quantification of proliferation of lymphocytes stimulated by anti-CD3 antibodies or phytohaemagglutinin using a diluted whole blood method [3–5]. T cell proliferative responses at an early stage of disease were a mean of 55% of those of concurrent non-infected controls and declined further in the patients who were within several months of progression to AIDS [4]. In these patients, a significant decline in T cell proliferative response was an independent marker of progression to AIDS. In other homosexual men with a normal blood level of CD4 T cells, despite chronic infection with HIV, there were decreased proliferative responses of CD4 and CD8 T cells to anti-CD3 antibody, depressed CD4 T cell help to B cells in mitogen-driven production of immunoglobulins, and diminished cytotoxic CD8 T cell activity against influenza virus [6]. Current studies have shown that successful anti-retroviral therapy only partially reconstitutes T cell tissue pools and T cell functions, and fails to reverse lymphopoietic exhaustion [7,8]. Recent investigation of proliferative responses T cell subsets in chronically HIV-infected homosexual men also has demonstrated two distinct mechanisms. Recruitment of naive CD4 T cells into proliferating pools was evoked principally by low levels of CD4 T cells, whereas proliferation of naive CD8 T cells was driven predominantly by HIV RNA [9]. There was corresponding evidence in these HIV-infected men for greater activation of CD4 than CD8 T cells by interferon (IFN)-α and interleukin (IL)-7.
HIV-infected adults over age 50 years progress to AIDS more rapidly than younger patients, but no therapeutic modalities target this difference [10]. Evidence of accelerated immunosenescence in chronically HIV-infected patients, as well as more rapid ageing of some other organ systems, has now been found [11–17]. The occurrence of a profile of polypathology in chronic HIV infection resembling that of ageing suggests that a drug capable of preferentially enhancing T cell function in elderly people should be evaluated for its effects on T cell functions in middle-aged HIV-infected individuals.
Lenalidomide, an analogue of thalidomide used to treat some immune cell malignancies, attains mean maximal levels of 100–400 nM in these protocols [18,19]. At similar levels of 30–1000 nM, lenalidomide enhanced T cell functions much more in older than in younger healthy subjects. Generation of IL-2 and IFN-γ by T cell receptor-stimulated T cells of young subjects (aged 21–40 years) were increased maximally by up to 16-fold and threefold, respectively [20]. The same concentrations of lenalidomide enhanced IL-2 and IFN-γ generation by stimulated T cells of older subjects (ages > 65 years) much more, with respective maximal increases of up to 115-fold and sixfold. Lenalidomide also enhanced proliferation and suppressed apoptosis of stimulated T cells from older subjects by IL-2-dependent mechanisms, and restored diminished T cell chemotactic responses to CCL21 chemokine and sphingosine 1-phosphate (S1P) [20]. Thalidomide and several other thalidomide analogues tested lacked this capacity to enhance T cell activation [20].
In the present study, IL-2 generation and chemotaxis to S1P and CCL21 all were impaired significantly for T cells of chronically HIV-infected middle-aged men, despite adequate treatment that partially corrected blood levels of CD4 T cells. Concentrations of lenalidomide that were highly immunostimulatory for T cells of healthy older subjects augmented significantly these defective functions of T cells from the HIV-infected men. Lenalidomide also augmented these functions of T cells from both healthy HIV-negative men, studied at an abnormally low CD4 : CD8 ratio in vitro, and chronically CD4 T lymphocytopenic HIV-negative men, suggesting that a major requirement for lenalidomide responsiveness is CD4 lymphocytopenia.
Materials and methods
Patient selection and evaluation
Six male HIV-infected subjects with ages ranging from 30 to 56 years were studied concurrently with healthy HIV-negative control men matched by gender and age. All HIV-infected subjects were on stable anti-retroviral therapy (ART) programmes for at least 9 months, taking no additional immunoactive drugs, and without active infection or malignancy. HIV-infected patients also were excluded for detectable blood HIV RNA, hepatitis C seropositivity, blood CD4 count less than 330 per microlitre at the time of study, ongoing cancer therapy, renal failure requiring haemodialysis or receipt of a vaccine within 1 month before the study. Three HIV-negative men aged 37–63 years with CD4-only T lymphocytopenia for a minimum duration of 6 months also were studied in parallel. These patients had abnormally frequent minor respiratory infections, but no AIDS-type serious infections. All subjects gave informed consent to participate in the study, which was approved by the University of California, San Francisco Committee on Human Research. Sixty ml of blood were collected once by standard venipuncture.
Isolation of T cells
Heparinized blood was diluted 1:1 (v : v) with calcium- and magnesium-free phosphate-buffered saline and 30 ml portions centrifuged on 10 ml cushions of Ficoll-Paque (GE Healthcare Life Sciences, Pittsburgh, PA, USA) for 30 min at 400 g to resolve mixed mononuclear leucocytes at the interface from other blood cells, as described [21]. The total population of T cells was obtained at >96 % purity by immunomagnetic depletion of all non-T cell mononuclear leucocytes, including natural killer (NK) cells, with an antibody cocktail-based negative-selection kit (Miltenyi Biotec, Auburn, CA, USA). CD4 and CD8 T cells were isolated from mixed T cells by positive immunomagnetic bead chromatography procedures (Miltenyi Biotec).
Quantification of cytokines by enzyme-linked immunosorbent assays (ELISAs)
Purified T cells were suspended at 106/ml in RPMI-1640 with 10% fetal bovine serum, 100 U of penicillin G and 50 µg/ml of streptomycin (UCSF Cell Culture Facility) and 1-ml aliquots cultured in wells of 24-well plates (Corning Life Sciences, Lowell, MA, USA) that had been pre-incubated with 1 µg each of anti-human CD3 plus anti-human CD28 antibodies (BioLegend, Inc., San Diego, CA, USA), as described previously [22]. Lenalidomide (sources in [20]) stock solution in dimethylsulphoxide (DMSO) was then diluted into medium so that the final concentration of DMSO was <1% (v : v). The concentrations of IL-2 after 24 h and IFN-γ after 48 h in duplicate aliquots of T cell supernates were quantified at respective dilutions of 1:5 and 1:300 with colorimetric ELISAs (MiniKits from Thermo Scientific-Pierce Biotechnology, Inc., Rockford, IL, USA). Colour intensity was determined in a VersaMax Microplate Reader (Sunnyvale, CA, USA) and the value for each point calculated from the average of the duplicate results.
Assessment of T cell chemotaxis
T cells were incubated overnight in RPMI-1640–penicillin G–streptomycin medium with 10% charcoal- and dextran-extracted fetal bovine serum (CD–FBS; UCSF Cell Culture Facility) to deplete T cell S1P. Transwell plate permeable upper inserts with a 5-µm-diameter pore filter (Corning Life Sciences) were pre-incubated overnight at 4°C in human type IV collagen, washed and dried as described [23]. T cells were pre-incubated with lenalidomide for 15 min at 37°C. Each of duplicate upper inserts received 106 T cells in 0·1 ml of CD–FBS–RPMI-1640 and was placed in a well containing 0·6 ml of CD–FBS–RPMI-1640 without (control) or with 10−7 M S1P (Sigma Chemical Co., St Louis, MO, USA) or 3 × 10−8 M CCL21 (Peprotech, Inc., Rocky Hill, NJ, USA). After incubation at 37°C for 4 h, the number of T cells in each lower compartment was determined by manual microscopic counting. All samples were blinded by coding and one investigator performed all counts. The results are expressed as a percentage of the initial number added to the upper insert.
Statistical evaluations
Data for each group were examined with the Kolmogorov–Smirnov test to confirm that they were distributed normally. The significance of differences between mean values in any series of studies was calculated by a two-sample t-test (GraphPad Software, La Jolla, CA, USA).
Results
Characteristics of study subjects
The age mean and ranges of the HIV-infected patients, CD4 T lymphocytopenic patients and control subjects were similar (Table 1). Each group of study subjects also had a diverse racial distribution. All HIV-infected patients were on a stable anti-HIV ART programme that eliminated detectable blood HIV RNA and raised their blood CD4 T cell levels to at least 330 per microlitre from a nadir below 200 per microlitre. The blood CD4 T cell levels of the CD4 T lymphocytopenic patients were similar to the nadir levels for the HIV-infected patients. CD4/CD8 T cell ratios were similarly low for the HIV-infected and CD4 T lymphocytopenic patients.
Table 1.
Study group characteristics
| CD4 level | CD4/CD8 | |||||||
|---|---|---|---|---|---|---|---|---|
| HIV duration | Treatment period | CD4 nadir | (at time of study) | |||||
| Group | Number of men | Age, mean (range) | Race | (mean, years) | (number/µl) | |||
| HIV-infected | 6 | 43 (30–56) | 2C, 2AA, 2H | 12 | 2·4 | 187 ± 112 | 840 ± 374 | 0·71 ± 0·50 |
| Control | 6 | 41 (25–54) | 3C, 2AA, 1A | |||||
| HIV-negative lymphopenia | 3 | 47 (37–63) | 1C, 1A, 1AA | 258 ± 42 | 0·70 ± 0·09 | |||
| Control | 3 | 49 (42–60) | 2C, 1A | |||||
C: Caucasian; AA: African American; H: Hispanic; A: Asian; HIV: human immunodeficiency virus. Values for CD4 and CD4/CD8 were determined within 4 weeks before or after the T cell functional studies, and are mean ± standard deviation; treatment period represents total duration; laboratory normal mean for CD4/CD8 is 1·7.
Impaired chemotaxis and IL-2 generation by T cells of HIV-infected patients
T cells from HIV-infected men had significantly lower chemotactic responses to both S1P and CCL21 than T cells from HIV-negative control subjects (Fig. 1a). The generation of IL-2, but not IFN-γ, also was significantly less for T cells from HIV-infected men than for control uninfected men (Fig. 1b).
Fig. 1.
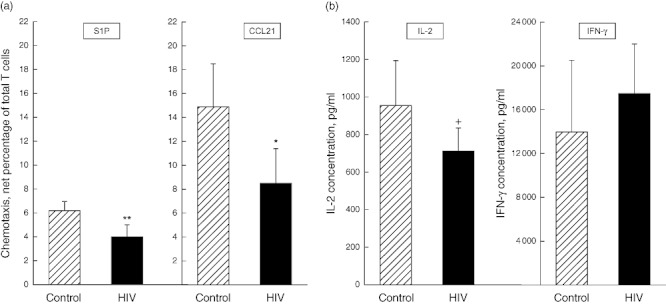
Functions of T cells from human immunodeficiency virus (HIV)-infected and non-infected control subjects. Each column and bar depicts the mean ± standard deviation of (a) the chemotactic responses of T cells to 10−7 M sphingosine 1-phosphate (S1P) and 3 × 10−8 M chemokine CCL21, as a percentage of the 106 T cells introduced initially into the upper chamber, and of (b) interleukin (IL)-2 and interferon (IFN)-γ concentrations after 24 h and 48 h, respectively, in culture media of activated T cells. The statistical significance of differences between values for T cells from control and HIV-infected subjects by t-test is shown; +P < 0·05; *P < 0·01; **P < 0·001.
Lenalidomide correction of impaired chemotaxis and generation of IL-2 by T cells from HIV-infected patients
Lenalidomide at 100 nM and 1 µM enhanced chemotaxis of T cells from HIV-infected men significantly to both S1P and CCL21, but had no effect on chemotaxis of T cells from healthy control HIV-negative men (Fig. 2a). The extent of lenalidomide-induced increases in chemotaxis for T cells of HIV-infected men raised the levels elicited by both stimuli to or above those seen for T cells of control uninfected men (Fig. 1a). Lenalidomide at 10 nM to 1 µM significantly augmented the generation of IL-2 by T cells from HIV-infected men to a maximum of over 300% (Fig. 2b). For T cells from healthy control HIV-negative men, 100 nM to 1 µM lenalidomide augmented the generation of IL-2 significantly, but only to a mean maximum of 175%. At all concentrations of lenalidomide, the increases in IL-2 levels generated by T cells from HIV-infected men were significantly greater (P < 0·01) than those for T cells from healthy control HIV-negative men. The same concentrations of lenalidomide had no effect on generation of IFN-γ by T cells from healthy control HIV-negative men and only 300 nM and 1 µM lenalidomide augmented by a mean maximum of just over 125% the generation of IFN-γ by T cells from HIV-infected men (Fig. 2b).
Fig. 2.
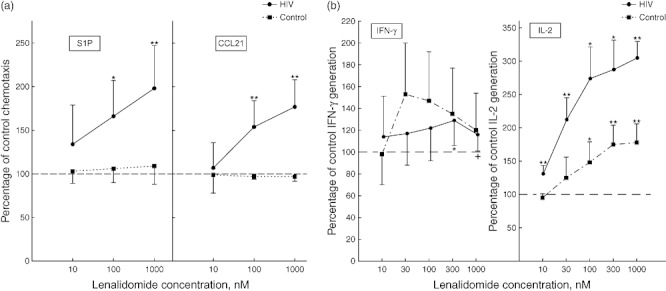
Effects of lenalidomide on functions of T cells from human immunodeficiency virus (HIV)-infected and non-infected control subjects. Each point and bar depicts the mean ± standard deviation of (a) the chemotactic responses of lenalidomide-treated T cells of that group to 10−7 M sphingosine 1-phosphate (S1P) and 3 × 10−8 M chemokine CCL21 and (b) interleukin (IL)-2 and interferon (IFN)-γ concentrations in media of lenalidomide-treated activated T cells of that group; for both frames as a percentage of the level for the same T cells without lenalidomide treatment (100%). Statistical significance is shown as in Fig. 1.
Contributions of depressed CD4 : CD8 T cell ratio to diminished T cell chemotaxis and generation of IL-2 in HIV-infected patients
The CD4 : CD8 T cell ratio was lower for HIV-infected men than control HIV-negative men (Table 1). A potential role for this compositional abnormality of T cell subsets in their impaired functions was investigated with T cells isolated from three HIV-negative healthy middle-aged men. CD4 and CD8 T cells obtained by immunomagnetic bead chromatography from each donor were remixed at ratios of 2, 1 and 0·5. T cell chemotaxis to S1P, but not CCL21, decreased with CD4 : CD8 ratio and was significantly lower at a ratio of 0·5 than 2 (Fig. 3). Lenalidomide increased T cell chemotaxis to S1P and CCL21 modestly, with direct concentration-dependence and relationship to the CD4 : CD8 ratio. Significant enhancement of T cell chemotaxis to either stimulus was observed only at the two lower ratios of CD4 : CD8 T cells and at 100 nM or 1 µM lenalidomide. The extent of maximal enhancement of T cell chemotaxis to either stimulus by lenalidomide was lower than that seen for T cells of HIV-infected men (Fig. 2a).
Fig. 3.
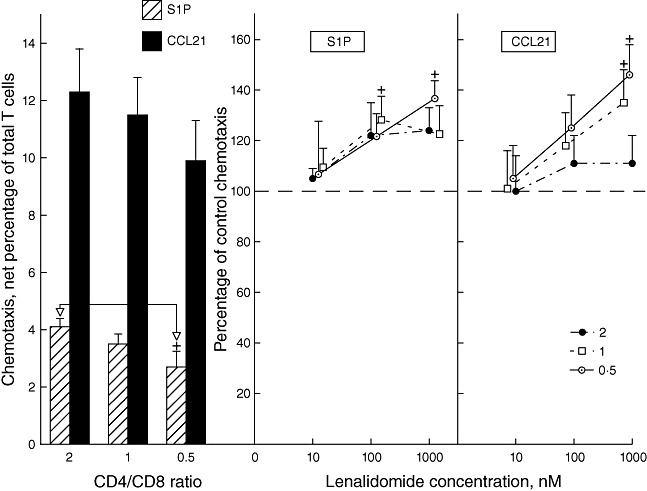
Influence of CD4 : CD8 T cell ratio on healthy male human T cell chemotaxis and the effects of lenalidomide on T cell chemotaxis. Left-hand frame: each column and bar depicts the mean ± standard deviation (s.d.) of the chemotactic responses of T cells from three human immunodeficiency virus (HIV)-negative healthy male subjects to 10−7 M sphingosine 1-phosphate (S1P) and 3 × 10−8 M chemokine CCL21, as a percentage of the 106 T cells introduced initially into the upper chamber. The statistical significance of one difference in chemotaxis to S1P between T cells at a CD4/CD8 ratio of 0·5 compared to those at a ratio of 2 is shown by+P < 0·05. Right-hand frames: each point and bar depicts the mean ± s.d. of the chemotactic responses of lenalidomide-treated T cells from the three subjects to 10−7 M S1P and 3 × 10−8 M CCL21, as a percentage of the chemotaxis of the same T cells without lenalidomide treatment (100%). Statistical significance of differences between chemotaxis of lenalidomide-treated and control T cells is shown as in Fig. 1.
The generation of IL-2, but not IFN-γ, decreased with CD4 : CD8 ratio and was significantly lower at a ratio of 0·5 and 1 than 2 (Fig. 4). The magnitude of decrease in IL-2 generation when the CD4 : CD8 ratio dropped from 2 to 0·5 was similar to that found between IL-2 generation by T cells of HIV-negative control men and HIV-infected men (Fig. 1b). Enhancement of IL-2 generation was observed at all concentrations of lenalidomide and all CD4 : CD8 T cell ratios, with no significant differences between levels of augmentation for any of the conditions. The magnitude of maximal enhancement of IL-2 generation by lenalidomide was similar to that seen for T cells of HIV-negative control men, but at any CD4 : CD8 ratio did not attain the level of augmentation found with T cells of HIV-infected men (Fig. 2b). A modest suppression of IFN-γ generation by lenalidomide was significant only at one point, due largely to less variability in the data at this concentration (Fig. 4).
Fig. 4.
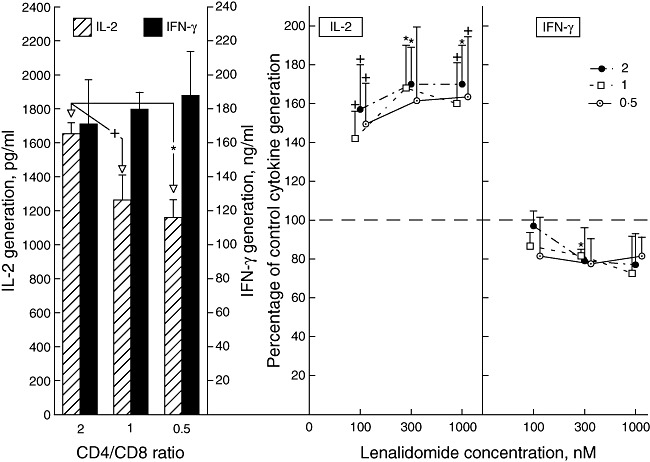
Influence of CD4 : CD8 T cell ratio on healthy male human T cell generation of interleukin (IL)-2 and interferon (IFN)-γ and the effects of lenalidomide on T cell cytokine generation. Left-hand frame: each column and bar depicts the mean ± standard deviation (s.d.) of the concentrations of IL-2 and interferon (IFN)-γ attained by activated T cells from three male subjects. The statistical significance of differences in IL-2 concentrations between T cells at a CD4 : CD8 ratio of 0·5 and 1 compared to that at a ratio of 2 is shown by the same symbols as in Fig. 1. Right-hand frames: each point and bar depicts the mean ± s.d. of the cytokine concentration achieved by lenalidomide-treated activated T cells from the three subjects as a percentage of that by the same T cells without lenalidomide treatment (100%). Statistical significance of differences between cytokine generation by treated and control T cells is shown as in Fig. 1.
Lenalidomide enhancement of impaired chemotaxis and generation of IL-2 by T cells from HIV-negative CD4 T lymphocytopenic patients
Mean [± standard deviation (s.d.)] chemotaxis of T cells from CD4 T lymphocytopenic patients to S1P was 4·4 ± 1·1% compared to 6·7 ± 1·2% for T cells of concurrently studied control subjects (P < 0·05), and for CCL21 was 10·6 ± 2·1% compared to 16·9 ± 3·3% for controls (P < 0·01). Impaired chemotaxis of T cells from CD4 T lymphocytopenic patients to S1P was enhanced significantly by 30–1000 nM lenalidomide (Fig. 5) to a mean maximum similar to that attained for T cells of HIV-infected patients (Fig. 2a). Impaired chemotaxis of T cells from CD4 T lymphocytopenic patients to CCL21 was enhanced significantly by 300 and 1000 nM lenalidomide (Fig. 5) to a mean maximum less than that attained for T cells of HIV-infected patients (Fig. 2a).
Fig. 5.
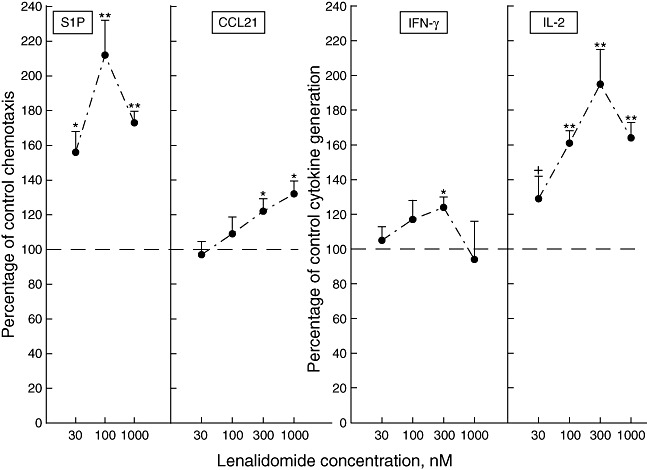
Effects of lenalidomide on functions of T cells from human immunodeficiency virus (HIV)-negative CD4 T lymphocytopenic subjects. Each point and bar depicts the mean ± standard deviation of the chemotactic responses of lenalidomide-treated T cells to 10−7 M sphingosine 1-phosphate (S1P) and 3 × 10−8 M chemokine CCL21 (left-hand frames), and of interleukin (IL)-2 and interferon (IFN)-γ concentrations in media of lenalidomide-treated activated T cells (right-hand frames); all values are a percentage of the level for the same T cells without lenalidomide treatment (100%). Statistical significance is shown as in Fig. 1.
Mean (± s.d.) generation of IL-2 by T cells from CD4 T lymphocytopenic patients was 71 ± 14% of that by T cells of concurrently studied control subjects (P < 0·05), whereas no difference between the groups was found for generation of IFN-γ. Impaired IL-2 generation by T cells from CD4 T lymphocytopenic patients was enhanced significantly by 30 nM to 1 µM lenalidomide (Fig. 5) to a mean maximum less than that attained for T cells of HIV-infected patients (Fig. 2b). Impaired IFN-γ generation by T cells from CD4 T lymphocytopenic patients was enhanced only up to a mean maximum of 124% by 300 nM lenalidomide (Fig. 5), as had been seen with T cells of HIV-infected patients (Fig. 2b).
Discussion
The present findings extend earlier observations of reduced in-vitro proliferative responses of T cells from HIV-infected patients to similarly diminished chemotaxis evoked by two distinct stimuli and to generation of IL-2, but not IFN-γ (Fig. 1). The extent of depression of both types of functions was significant, despite the small number of subjects and resultant potential statistical bias of the findings. The abnormally low in-vitro chemotactic responses to two stimuli involved in T cell trafficking implies that T cell patrolling of lymphoid and other tissues in vivo is impaired in HIV-infected patients. Therapeutically attainable concentrations of lenalidomide enhanced functions of T cells from HIV-infected patients significantly more than those of T cells from HIV-negative control subjects and thereby corrected the abnormalities in both chemotaxis and IL-2 generation (Fig. 2). The most effective concentrations of lenalidomide studied in vitro were similar to those attained in therapy of several types of cancers and T cell exposure times would also be similar to those in treatment for in-vitro cytokine generation, but less for chemotaxis.
The possibility that a depressed CD4 : CD8 T cell ratio in HIV-infected patients may contribute to impaired immune functions of the total T cell population was examined initially with mixtures of T cells prepared at CD4 : CD8 ratios encompassing normal levels and the lower levels found in HIV-infected patients (Table 1). Qualitatively similar, but lesser, decrements in chemotaxis to S1P but not CCL21 and in generation of IL-2 but not IFN-γ were elicited by conducting functional analyses of T cells from healthy subjects at ratios of CD4 : CD8 T cells characteristic of HIV-infected patients (Figs 3 and 4). Thus, the diminished immune functions of T cells of HIV-infected patients may, in part, be attributable to decrements in the ratio of CD4 : CD8 T cells.
Abnormally low levels of chemotaxis to S1P and sometimes CCL21 also have been documented for T cells from patients with CD4 and/or CD8 T lymphocytopenia who also have depressed ratios of CD4 : CD8 T cells (Table 1) [23]. T cells from a group of three male patients with CD4 T lymphocytopenia, who were age-matched with the HIV-infected men, had similar in-vitro abnormalities of chemotaxis and IL-2 generation to those of the HIV-infected patients (Fig. 5). These impairments of T cell functions were corrected by lenalidomide to the same extent as those of T cells from HIV-infected patients, except that the enhancement of IL-2 generation was less than that found for the T cells of matched HIV-infected patients (Fig. 5). Thus, it is possible that the homeostatic corrective mechanisms evoked by low T cell counts may be responsible for some of the similar alterations in T cell immune functions in both conditions. This same possibility was invoked to explain the difference in mechanisms between mobilization of CD4 and CD8 T cells in HIV infections [9].
Experienced investigators of HIV infections in humans have observed accelerated ageing in patients with AIDS [13–15]. Numerous AIDS-related morbidities, including immune depletion, despite successful therapy that controls HIV infection and immune activation leading to chronic inflammation, are also characteristics of human immunosenescence without HIV infection [24–28]. Individuals with HIV infections and healthy elderly people both have similar changes in T cell composition, including low ratios of CD4 : CD8 T cells and of naive : memory T cells, as well as expanded CD57 and CD28- CD8 subsets [15]. Both groups also have similar changes in T cell functions, including decreased proliferative responses and IL-2 generation to stimulation, as well as restricted T cell antigen receptor repertoire and impaired T cell responses to vaccines. Thus, it seems possible that the shared responses to low concentrations of lenalidomide of T cells from both HIV-infected middle-aged patients and healthy elderly subjects may be attributable to the same mechanisms [20]. Accelerated senescence also appears to be a predictor of non-AIDS-related co-morbidities in HIV-infected men [29]. Some components of ART contribute to chronic inflammation, inflammation-associated manifestations, such as the lipodystrophic phenotype, and widespread cellular senescence [30–32]. However, the similarity of T cell immune abnormalities in HIV-infected and HIV-negative CD4 T lymphocytopenic patients argues against a major role of these drugs as contrasted with other immune mechanisms.
Acknowledgments
This research was supported by endowment funds of the Jewish Home of San Francisco and the intramural research programme of the National Institute on Aging. The authors are grateful to Judith H. Goetzl for preparation of the figures and tables and for editorial suggestions.
Disclosure
Authors have no disclosures to report.
References
- 1.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 2.Smit C, Geskus R, Walker S, et al. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS. 2006;20:741–9. doi: 10.1097/01.aids.0000216375.99560.a2. [DOI] [PubMed] [Google Scholar]
- 3.Miedema F, Petit AJ, Terpstra FG, et al. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J Clin Invest. 1988;82:1908–14. doi: 10.1172/JCI113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roos MT, Miedema F, Koot M, et al. T cell function in vitro is an independent progression marker for AIDS in human immunodeficiency virus-infected asymptomatic subjects. J Infect Dis. 1995;171:531–6. doi: 10.1093/infdis/171.3.531. [DOI] [PubMed] [Google Scholar]
- 5.Bindels PJ, Krol A, Roos M, et al. The predictive value of T cell function in vitro and pre-AIDS zidovudine use for survival after AIDS diagnosis in a cohort of homosexual men in Amsterdam. J Infect Dis. 1995;172:97–104. doi: 10.1093/infdis/172.1.97. [DOI] [PubMed] [Google Scholar]
- 6.Shearer GM, Payne SM, Joseph LJ, Biddison WE. Functional T lymphocyte immune deficiency in a population of homosexual men who do not exhibit symptoms of acquired immune deficiency syndrome. J Clin Invest. 1984;74:496–506. doi: 10.1172/JCI111447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lange CG, Lederman MM. Immune reconstitution with antiretroviral therapies in chronic HIV-1 infection. J Antimicrob Chemother. 2003;51:1–4. doi: 10.1093/jac/dkg071. [DOI] [PubMed] [Google Scholar]
- 8.Sauce D, Larsen M, Fastenackels S, et al. HIV disease progression despite suppression of viral replication is associated with exhaustion of lymphopoiesis. Blood. 2011;117:5142–51. doi: 10.1182/blood-2011-01-331306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catalfamo M, Wilhelm C, Tcheung L, et al. CD4 and CD8 T cell immune activation during chronic HIV infection: roles of homeostasis, HIV, type I IFN, and IL-7. J Immunol. 2011;186:2106–16. doi: 10.4049/jimmunol.1002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rickabaugh TM, Kilpatrick RD, Hultin LE, et al. The dual impact of HIV-1 infection and aging on naive CD4 T-cells: additive and distinct patterns of impairment. PLoS ONE. 2011;6:e16459. doi: 10.1371/journal.pone.0016459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22:1615–24. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grunfeld C, Delaney JA, Wanke C, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23:1841–9. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep. 2010;7:69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- 14.Appay V, Fastenackels S, Katlama C, et al. Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS. 2011;25:1813–22. doi: 10.1097/QAD.0b013e32834640e6. [DOI] [PubMed] [Google Scholar]
- 15.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–55. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan RC, Sinclair E, Landay AL, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2010;203:452–63. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capeau J. Premature aging and premature age-related comorbidities in HIV-infected patients: facts and hypotheses. Clin Infect Dis. 2011;53:1127–9. doi: 10.1093/cid/cir628. [DOI] [PubMed] [Google Scholar]
- 18.Keizman D, Zahurak M, Sinibaldi V, et al. Lenalidomide in nonmetastatic biochemically relapsed prostate cancer: results of a phase I/II double-blinded, randomized study. Clin Cancer Res. 2010;16:5269–76. doi: 10.1158/1078-0432.CCR-10-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmeister CC, Yang X, Pichiorri F, et al. Phase I trial of lenalidomide and CCI-779 in patients with relapsed multiple myeloma: evidence for lenalidomide-CCI-779 interaction via P-glycoprotein. J Clin Oncol. 2011;29:3427–34. doi: 10.1200/JCO.2010.32.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang MC, Greig NH, Luo W, et al. Preferential enhancement of older human T cell cytokine generation, chemotaxis, proliferation and survival by lenalidomide. Clin Immunol. 2011;138:201–11. doi: 10.1016/j.clim.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang MC, Patel K, Taub DD, Longo DL, Goetzl EJ. Human CD4– 8– T cells are a distinctive immunoregulatory subset. FASEB J. 2010;24:2558–66. doi: 10.1096/fj.09-153148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goetzl EJ, Huang MC, Kon J, et al. Gender specificity of altered human immune cytokine profiles in aging. FASEB J. 2010;24:3580–9. doi: 10.1096/fj.10-160911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goetzl EJ, Schwartz JB, Huang MC. Defective T cell chemotaxis to sphingosine 1-phosphate and chemokine CCL21 in idiopathic T lymphocytopenia. J Clin Immunol. 2011;31:744–51. doi: 10.1007/s10875-011-9554-2. [DOI] [PubMed] [Google Scholar]
- 24.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–95. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tien PC, Choi AI, Zolopa AR, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55:316–22. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyons JL, Uno H, Ancuta P, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr. 2011;57:371–9. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–6. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 30.Lagathu C, Eustace B, Prot M, et al. Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir Ther. 2007;12:489–500. [PubMed] [Google Scholar]
- 31.Lefevre C, Auclair M, Boccara F, et al. Premature senescence of vascular cells is induced by HIV protease inhibitors: implication of prelamin A and reversion by statin. Arterioscler Thromb Vasc Biol. 2010;30:2611–20. doi: 10.1161/ATVBAHA.110.213603. [DOI] [PubMed] [Google Scholar]
- 32.Guaraldi G, Stentarelli C, Zona S, et al. Lipodystrophy and anti-retroviral therapy as predictors of sub-clinical atherosclerosis in human immunodeficiency virus infected subjects. Atherosclerosis. 2010;208:222–7. doi: 10.1016/j.atherosclerosis.2009.06.011. [DOI] [PubMed] [Google Scholar]


