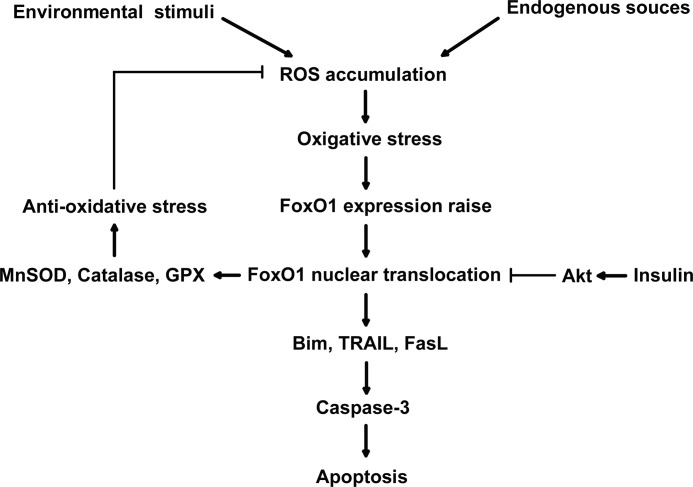
FIGURE 8.
A model of the role of FoxO1 in oxidative stress-induced apoptosis in mouse granulosa cells. When ROS is generated either by endogenous sources like aerobic metabolism and ischemia or by environmental stress like ionizing radiation and UV light, the excessive ROS accumulation results in oxidative stress in the cells. As a pivotal sensor of intracellular ROS, FoxO1 function is enhanced by both FoxO1 expression up-regulation and nuclear translocation, therefore activating the transcription of FoxO1 target genes, including either proapoptosis or stress-resistant genes. The proapoptotic genes transfer the stress signal through mitochondrial pathway and finally initiate caspase-3 activity and cell apoptosis. But the oxidative stress-resistant genes serve to reduce the cellular ROS level, which may provide negative feedback to balance the FoxO1 expression during oxidative stress. Independently, insulin signaling can reduce the FoxO1 function via the PKB/AKT pathway phosphorylating FoxO1 and prohibiting the FoxO1 nuclear localization, thereby protecting granulosa cells from oxidative stress-induced apoptosis.