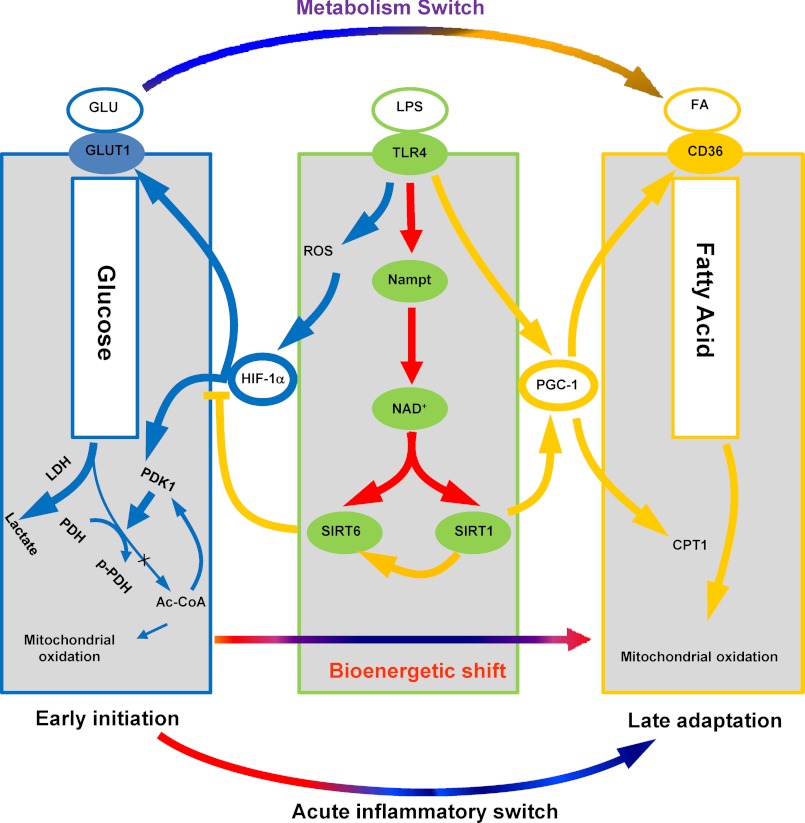
FIGURE 9.
Alignment of NAD+-dependent metabolic and inflammatory switching after acute TLR4 responses and during sepsis. When sepsis is ignited by TLR responses, the high energy required for the early inflammatory response generates reactive oxygen species (ROS), which stabilizes HIF-1α protein and activates its transcription of glycolysis genes in concert with NF-κB p65 and its activation of proinflammatory genes. HIF-1α increases glycolysis, but inhibits mitochondrial glucose oxidation. As the early inflammatory response changes to late adaptation, glucose metabolism switches to increased fatty acid flux and enhanced fatty acid mitochondrial oxidation. Both the metabolic and adaptive switches require activation of Nampt and NAD+ sensors SirT1 and SirT6. SirT6 represses glucose metabolism by epigenetically silencing the HIF-1α path. SirT1 supports fatty acid oxidation by activating the PGC-1 path, which also promotes mitochondrial biogenesis and restores homeostasis in sepsis survivors.