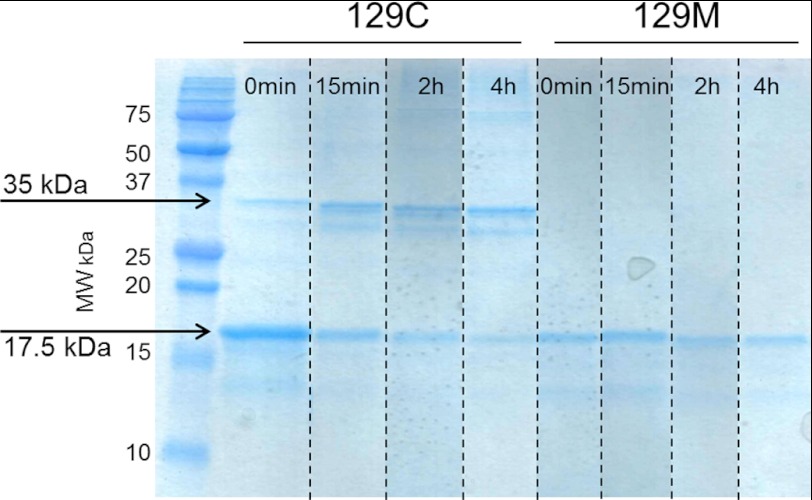
FIGURE 3.
Non-reduced denaturing SDS-PAGE of M129C and WT variants. Samples taken at different time points during the fibril formation reaction were boiled in non-reducing SDS loading buffer and run on 18% PAGE gels using SDS buffer, and the gel was stained with Coomassie Brilliant Blue. The M129C mutant shows the presence of a 35-kDa band (dimer) that increases over time as the 17.5-kDa band (monomer) decreases. For the WT (129M) variant only, the PrP monomer was detected at all time points. The image presented is a composite from duplicate samples run on the same gel (as indicated by dashed lines). MW, molecular weight.