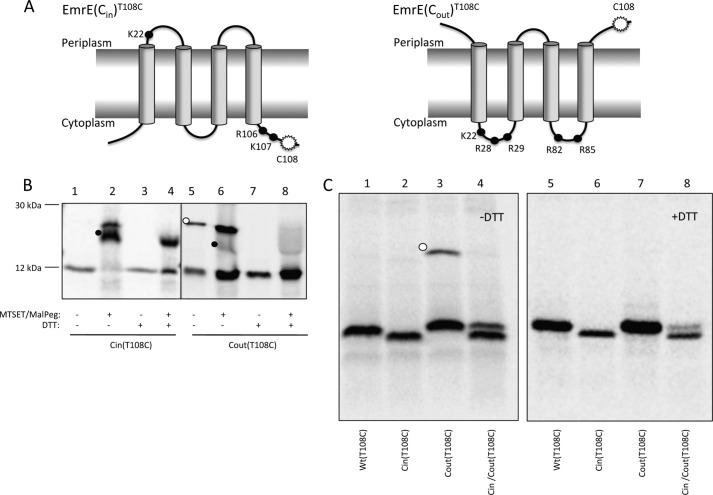
FIGURE 1.
Topology mapping of EmrE variants by cysteine accessibility. A, schematic representations of the oppositely orientated EmrE variants, EmrE(Cin) and EmrE(Cout). The mutations in EmrE(Cin) are R29G, R82S, and S107K, and in EmrE(Cout) T28R, L85R, and R106A. Black dots represent positively charged residues. The white starburst represents the position of the single cysteine Cys108 used for topology determination by MTSET/Mal-PEG treatment of whole cells. B, cysteine labeling of EmrE(Cin)T108C and EmrE(Cout)T108C. Periplasmic cysteines were blocked by treatment of whole cells with membrane-impermeable MTSET, cells were lysed, and remaining free cysteines were reacted with Mal-PEG that causes a size shift (black dots). The disulfide-bonded dimer of EmrE(Cout)T108C is indicated by a white dot. C, SDS-PAGE gels showing spontaneous in vivo cysteine cross-linking of EmrE(Cout)T108C (lane 3, white dot) but not of EmrET108C, EmrE(Cin)T108C, or co-expressed EmrE(Cin)/EmrE(Cout)T108C (lanes 1, 2, and 4). Lanes 5–8 shows the same samples after reduction with DTT. Note that EmrE(Cin) migrates faster than EmrE(Cout) and EmrE(WT) on SDS-PAGE gels.